
|
|
All About Oil
The subjects of which oils are best to use in model engines and how much should be used seem to flare up into a heated debate every few years or so. The main bone of contention is generally centered around the pros and cons of using castor oil or synthetic oils. Both have their staunch adherents, many of whom seem to hold very strong views on the subject. Proponents of both types of lubricant tend to extol their virtues while ignoring or discounting their disadvantages. It was exactly the same during the 1980's when I was road-racing classic two-stroke motorcycles - the 5% castor oil crowd (of whom I was one) were endlessly debating with the 2% synthetic lobby! I have to say in passing that my camp won that argument decisively, based on the frequency with which we observed members of the other camp sitting disconsolately at trackside watching us race on while they contemplated their seized engines! The most amazing aspect of that situation was the tenacity withwhich they continued to maintain their views in the face of the evidence - it was never the oil which had let them down, but rather some other factor............! The psychologists call this well-recognized But that was in a full-sized racing engine context. When it comes to model engine lubricants, the same factors may or may not apply. In that context, the proponents of synthetic oils cite their far less “messy” operation and reduced tendency to generate engine deposits, also frequently claiming on unspecified evidence that they lubricate an engine just as effectively as castor oil. With respect to the latter point, any evidence to the contrary is generally either ignored or dismissed. Proponents of castor oil (like myself, I freely admit!) are forced to acknowledge the “messier” operation that results from the use of this lubricant as well as its increased tendency to generate engine deposits, but are prepared to accept that as the price to be paid for what they consider to be superior lubrication, particularly at high temperatures and extreme pressures. So who’s right?!? Well, as usual in cases such as this, both sides have valid points to support their arguments - there are pros and cons in relation to the use of either type of lubricant. Depending on the operating conditions to which the oil will be subjected, the plain fact is that either lubricant can work very well. The extent to which it does so will depend very largely upon the operating conditions to which it will be subjected. In the end, it comes down to a few fundamental points:
In the past, very little of this sometimes heated debate has been informed by any meaningful scientific data. For that reason, I was delighted when my good mate Maris Dislers drew my attention to a scientifically-based article by noted Danish team-race expert Luis Petersen on the subject of the oil types and content typically used in model engine fuels, specifically those employed in the demanding application of F2C team racing, in which engines run at full power for extended periods using fuels having very low oil contents. This article appeared in issue 3-2007 of the excellent Swedish control-line periodical LINA, although I believe that the tests reported in the text were conducted some years prior to that. Luis’s observations are always well worth considering – I've re-published his work on testing of 1970’s team-race engines elsewhere on this site. The main significance of his article on oils was its presentation of the results of a number of scientific tests on differing oil types and various oil proportions. To my knowledge, such work had never previously been published in a model engine context. The ever-helpful Maris took the trouble to translate the article as best he could and send it along. We are all greatly in his debt for doing so. While I found some of the comments quite fascinating, especially the graphs based upon testing of an actual engine, I felt that the article did not go far enough into certain aspects of this very important subject. Accordingly, rather than simply present a translation of Luis’s article, I decided to write my own article, citing Luis as one of my key references. I hereby do so, with very sincere thanks to Luis and LINA Editor Ingemar Larsson for making this information available for study.
Bert’s commentary was featured on George Aldrich’s web-site, which was taken down following George’s death in 2001. Thankfully, Maris Dislers took a scan of the article while it was still available and was kind enough to pass the text along to me for consultation during the preparation of the present article. Once again, we are greatly indebted to Maris for his kindness in this regard. There's also a good deal of authoritative information on oil technology available these days on the Web if you take the trouble to look. The time is long past when this discussion can legitimately be based upon gut feelings and emotions rather than hard data. A number of such sources were located and consulted during the preparation of this article. Now, having acknowledged our primary sources of technical data, let’s take an in-depth look at this issue on the basis of the wealth of information now available. The Functions of Oil To begin this little treatise, it’s probably as well to ensure that the function of the oil in model engine fuel is clearly understood. In point of fact, there are actually three such functions:
Obviously, the first of the above functions is the most important since it will have a direct bearing on the engine’s performance and longevity. The second function only becomes really critical in an engine that is fairly well worn – in an engine in good condition, only relatively small amounts of oil are required to meet this need provided a certain degree of viscosity (resistance to flow) is maintained at operating temperatures. The third function is one that is often overlooked, yet it has quite a high importance in terms of preventing a build-up of particulates in the engine which could promote undue wear. It’s highly desirable to have a sufficient continuous flow of oil through and out of the system to carry any particulates away in suspension and thus ensure that no such build-up can occur. Historical Background
Similar mineral-based lubricants were subsequently used in model diesel fuel during the pioneering era around WW2, since ether, kerosene and mineral oil are mutually miscible thanks to the powerful solvent properties of ether. Indeed, a high-quality mineral oil remains quite suitable for use in older-style slow-revving diesels. When the glow-plug engine arrived in the latter part of the 1940’s, it became necessary to use an oil that was compatible with methanol. This is when vegetable oil, notably castor oil, really came to prominence. Following the 1948 arrival on the scene of the high-performance glow-plug motor, more complex fuel mixtures quickly evolved which contained other fuel constituents such as nitromethane and nitrobenzene to release extra power. It was soon found that when the concentration of nitromethane exceeded 40-50%, the mixture became unstable since the oil would no longer remain in solution. Hence it became necessary to add other components such as propylene oxide for stabilization purposes.
It is these three categories of oil – mineral, vegetable and synthetic – which remain available for use in model engines today. Before considering the pros and cons of each, it’s well worth reviewing the issue of lubrication in general, since it is the most important of the three previously-listed functions that oil has to fulfil. In doing so, we will to a large extent be drawing upon the material presented in Luis Petersen’s previously-cited article. Lubrication Lubrication conditions in a model engine vary considerably for the different working components, both in terms of temperatures. materials and applied loadings. The most critical requirement is the maintenance of a constant oil film in the upper cylinder, which is of course the hottest part of the engine. This is made very challenging by the high temperatures, tight fits and low piston speeds typically encountered in the region of top dead center. To better understand the lubrication function of oil, it is necessary that we first take a look at the issue of friction. This after all is what we are trying to minimize in our engine in order to obtain its maximum possible performance and longevity.
A good example of the situation depicted in the above diagram is the piston/cylinder wall interface in a model engine, where the piston has to slide against the cylinder wall while exerting considerable side-thrust against it due to the angle of the con-rod. This side-thrust will create a certain amount of friction force which will resist the sliding of the piston in the cylinder bore and will have to be overcome in order to keep the engine running. Overcoming this friction force represents a direct loss of power, since the energy required to overcome friction is not available to generate power at the prop. The ratio between the friction force created and the side-thrust which created it is represented by the coefficient of friction for the two materials involved (piston and cylinder). Obviously, we want this figure to be as low as possible in order to minimize friction power losses. The coefficient of friction varies significantly depending on the materials involved. For example, ice on steel has an extremely low coefficient of friction, while rubber on pavement has a high coefficient of friction. Coefficients of friction range from near zero to greater than one – the mega-sticky tyres used by present-day motorcycle road-racers are examples of the latter, allowing lean angles well in excess of 60 degrees during cornering. Now we are in a position to describe the three categories of lubrication in which friction may be encountered.
This condition arises when two materials slide against one another in the total absence of any form of lubricant, forcing the two materials to interact directly with one another. A good example is the action of brake pads on brake discs, where we want to generate a lot of friction force in order to stop the vehicle by converting its kinetic energy into heat. Such a condition always involves both wear and the generation of heat regardless of the surface condition. Needless to say, this may be fine for brakes but is a highly undesirable condition in a model engine! Friction, heating and wear are all reduced with a finer surface finish, but some wear and heat generation are inevitable regardless. For model engine components, dry friction coefficients typically lie between 0.1 and 0.7 depending on the materials involved. In a model engine, we obviously want to avoid dry friction like the plague! Hence we try to ensure that all moving parts are well supplied with some sort of lubricant. In a situation where a film of lubricant is present, two distinct lubrication conditions may be encountered, as follows.
This situation causes friction development patterns to follow the hydrodynamic laws that apply to liquids. In this ideal case, the friction coefficient depends entirely on the lubricant viscosity (explained later), the lubricant film thickness and the relative speed between the moving surfaces – the two surface materials themselves have nothing to do with it. In model engines, coefficients of friction in this condition typically lie between 0.0001 and 0.005. We often refer to this liquid-related form of friction as "viscous drag". If the lubricant contains no form of abrasive particulates, there will be no wear of either of the two materials involved since they are not in direct contact with one another.
In this condition, the sustainable oil film thickness is less than the sum of the maximum heights of the asperities present on both surfaces. Consequently, there will be some degree of contact between the peaks of the asperities on the two surfaces, creating both wear and parasitic friction power losses. There will also be the generation of microscopic particles of the materials involved, which will require purging from the engine if they are not to contribute to accelerated wear. This is the situation most commonly encountered in a model engine. There are no fixed laws for this case. The degree to which the development of friction follows the laws for dry friction or liquid friction is determined by surface condition, sustainable film thickness and the fluid properties of the lubricating material. Total friction force will be the sum of the residual metal-to-metal friction component and the viscous drag imposed by the presence of the lubricant itself. Clearly this can vary considerably depending upon the specific situation. Friction coefficients in this condition typically lie between 0.005 and 0.100. Not inconsiderable, but still a major improvement over the dry friction condition! It should be clear by now that the more finely the two surfaces are finished (i.e., the lower the maximum asperity heights of the two surfaces), the smaller the oil film thickness required to manintain boundary or liquid lubrication conditions. This is why surface finish is such an important factor in the manufacture of working components in an engine. The only area in a model engine where we may encounter pure liquid lubrication is at the inlet, where stresses are relatively low and lubrication is ample given the constant inflow of cool fuel mixture. All other locations within the engine can be expected to operate under varying degrees of boundary lubrication. We can do nothing to prevent this condition from arising – we can only take what steps we can to minimize its effects. The critical thing is to ensure that we do not experience dry friction at any point. Next, let’s have a look at a few of the terms and criteria which we may encounter when considering our choice of oil for our model engine fuel. Criteria to Consider when Selecting an Oil To better understand the properties which are required in an effective lubricant, we can do no better than to paraphrase the comments on this issue which were included in Bert Streigler’s previously-cited article. Bert noted that for any fluid to act as a lubricant, it must first be "polar" enough to wet the moving surfaces – that is, it must have an affinity for the materials being lubricated. Next, it must have a high resistance to surface boiling and vaporization at the temperatures likely to be encountered. Ideally the fluid should also have "oiliness" to give it viscosity and film strength under operating conditions. This latter parameter is difficult to characterize, but it may be stated in general that the most "oily" and hence effective lubricants have a rather large molecular structure in chemical terms. There are of course exceptions to this rule – even water with its tiny molecule can be a good lubricant under the right conditions and with the right materials. Here are a few terms and criterial with which a degree of familiarity is beneficial when selecting an oil: Viscosity
The downside is of course that more viscous lubricants will impose greater levels of viscous drag upon moving parts during operation. However, that may be a small price to pay for decreased wear rates and surface fricrtion losses.
This value indicates how much the viscosity changes with temperature for a given oil. As a rule, oil tends to lose viscosity as the temperature rises. It should be clear from the previous discussion that in doing so, it inevitably loses some film strength and thickness. The attached diagram shows some typical graphs expressing the relationship between temperature and viscosity for a number of unspecified different oils. Film Thickness This parameter provides a representation of how effectively an oil can maintain a liquid film between two surfaces and thus prevent the onset of boundary lubrication. It is of course intimately related to viscosity. We have reviewed its significance in the previous section of this article. EP Properties In a lubricant context, the term EP means extreme pressure. It is often cited in connection with supplementary products (additives – see below) that are designed to form a kind of self-lubricating film at metal-to-metal boundaries to avoid undesirable wear. It has little relevance in a model engine context. Flash Point This is the lowest temperature at which an oil can vaporize sufficiently to form an ignitable mixture in air. However, at this temperature the combustion will not be sustainable - once the initial vapor charge is burned up, combustion will cease until more vapor forms. Even so, once an oil starts to vaporize (boil) in an engine, it has the potential to participate in the combustion process to some degree depending on its fire point (see below). In this condition it begins to lose many of its lubrication qualities. Fire Point This is defined as the temperature at which the vapor produced by a given oil will continue to burn for at least 5 seconds after ignition by an open flame. Put simply, it is the temperature at which oil vapor will be generated at a sufficient rate to make the combustion of that vapor become sustainable. It is normally considerably higher than the flash point, at which the oil will produce sufficient vepor to allow momentary ignition to occur but will produce vapor at an insufficient rate to sustain the fire. By the time the fire point is reached, the lubrication qualities of the oil will have been completely lost.
Some oil types cannot be mixed with other oils or certain types of fuel. It is important to understand any such issues which may be present with an oil that you are considering using. Internal Deposits These are deposits of solid materials that can accumulate in an engine as a result of its combustion and lubrication processes. They include such things as carbon deposits in the combustion area and varnish deposits on the hotter moving parts. An added factor is the generation of deposits such as gum in the engine during after-run storage. Different oils have their own individual characteristics when it comes to the creation of such deposits. SAE This commonly-encountered term is nothing more than an abbreviation for the Association that has developed the world-wide standards for classifying oils. It stands for the Society of Automotive Engineers. Types of Oil Having now discussed a number of applicable terms and criteria, let’s look at the types of oil which are available for our use. The range of available mineral, vegetable and synthetic oils is extremely and at times confusingly large. Oils such as Castrol M (a castor oil) and Castrol MSSR (a synthetic oil) are typical of the most commonly-used lubricants in model engines today. However, mineral oils still have their place as well. Let’s start with them.
This is a lubricant that is based upon a distilled fraction of some mineral source, notably petroleum. At one time this was the standard lubricant for model engines. It is readily miscible in both gasoline and ether-based fuels. SAE 70 mineral oil was typically used in spark ignition engines at a 3 to 1 gas/oil mixing ratio, as it still is today by classic and old time modellers who continue to use such engines. Castrol XXL was often recommended for use in early British diesels. However, finding a convenient source of SAE 70 oil has become rather problematic in recent years. A very acceptable latter-day substitute is AeroShell 120, a straight SAE 60 mineral oil blended from selected high viscosity index base oils specifically for use in full-sized piston aero engines. It contains no additives except for very small amounts of pour point depressant (which improves fluidity at very low temperatures) and an anti-oxidant. It is readily available from full-sized aviation support companies at most regional or municipal airports. Moreover, decades of further research have improved it to the point where it is a far superior lubricant to any SAE 70 or similar mineral oil that was available back in the '30's or '40's. Apart from its use in sparkies, AeroShell 120 also works well in older diesel engines from the pioneering era which operate at lower speeds, low temperatures and reduced compression ratios. It's also completely acceptable for use in latter-day replicas of such engines. However, although it remains readily available, mineral oil is seldom used as a lubricant in more recent model engines, particularly those of the high-performance variety. It is quite unsuitable for use in methanol-based fuels since it doesn't mix well. On the positive side, mineral oils do not form varnish deposits, nor do they cause the gumming-up of engines which are stored for long periods in between uses. In addition, they produce exhaust residues which are relatively easily cleaned up. As long as they are not over-stressed, they are actually excellent lubricants. On the negative side, although mineral oils have flash points which are very comparable to those of synthetic oils (typically around 400 degrees F (205 degrees C) for mineral oil), their fire points are not much greater than their flash points. Indeed, their auto-ignition temperatures (the temperatures at which they will spontaneously ignite in the absence of any ignition source) are generally only in the region of 608 degrees F (320 degrees C), at which point they cease to be a lubricant and become a fuel instead! This means that they become prone to participating in the combustion process more or less as soon as their flash point is reached, losing much of their lubrication effectiveness in the process. This tendency in turn makes them prone to encouraging the development of carbon deposits in the combustion chamber area. Consequently, mineral oils lack the high temperature and extreme pressure capabilities of Having said this, it's worth repeating that mineral oils in the SAE 50-60 viscosity range are perfectly satisfactory lubricants for model diesel engines of the "pioneering" type, since temperatures in such engines never approach the flash point of a good-quality mineral oil. The use of castor oil is probably a case of overkill in such engines. I have run many of my older diesels of the Mills type on mineral-based fuels, with perfectly satisfactory results over many hours of use. A tip that I can pass along is that diesel engines which may undergo a lengthy period of storage between starts will benefit from having a tank of mineral-based fuel run through them at reduced compression and on a rich needle prior to storage. Engines treated in this manner prior to storage will never gum up and require unsticking on the next occasion on which they are required to run. I have run engines which had been in storage for over 30 years following such treatment which still turned over freely after all that time. A highly recommended approach! Mineral oils also still find a specialized application in fixed-compression diesel fuel, which consists of straight ether and mineral oil. Because of the very cool running of the old fixed-compression diesels together with their relatively low speeds, SAE 40 mineral oil has been found to work very well in such engines. For more information, please see my separate article on fixed compression diesel operation to be found elsewhere on this site.
These are oils which are extracted from a plant source, generally in the form of triglycerides. A wide variety of potentially useful oils falls into this category, including olive, rapeseed (canola), coconut, soy, sesame and castor oil. Vegetable oils were widely used in full-sized aero engines of the WW1 era and have been widely used in model engines ever since the WW2 period when operation on methanol first became commonplace. The most commonly-used vegetable lubricant is of course castor oil. However, it is not the only potentially useful such lubricant. K&B formerly used epoxidized soybean oil in their high-nitro Supersonic Speed Fuel mix, also selling this lubricant to those wishing to make their own high-performance fuel. According to Luke Roy (co-producer of the classic record-breaking H&R Rattler racing engine), it was superior to castor oil, which is saying something! However, although it remains commonly used in the plastics industry, it is very difficult to obtain today in suitable quantities and grades for modelling use. Too bad - I'd like to try it myself ...... This leaves us with castor oil as the vegetable oil of choice among modellers. Brands available on the market in the past have included Castrol M, Castrol R, Duckhams, Prats, Bakers AA, Klotz Benol and Castrol R40, the latter two being polymerized forms of castor oil that are suitable for mixing with gasoline. These brands have generally incorporated additives to discourage carbon build-up and promote longer shelf life than the unadulterated 100% pure form. They were also de-gummed to discourage gumming-up during short-term storage. However, the ready availability of this material has become compromised in recent years thanks to the Ricin scare. In particular, hobby shops generally no longer stock Bakers AA or Klotz Benol castor oil as they often did formerly, at least in North America. You’ll have to look around, reflecting as you do so that once again we legitimate users pay the price for the illicit activities of others …… When you do find a In the past, castor oil was available in both lubricant and medical grades. The medical grade intended for internal use is still readily available in drug stores. However, it is quite unsuitable for use as a model engine lubricant – do not be tempted to try it! A useable substitute with which I have so far had completely satisfactory results is the 100% pure cold-pressed castor oil which is sold in beauty stores for external use as a skin emollient – for some reason, its availability has not so far been affected by the Ricin scare. Its main disadvantage is that it is not de-gummed, making it somewhat prone to gumming up a stored engine unless appropriate precautions are taken. If you decide to try this route, make sure that the label on the oil that you're buying specifically states that it is 100% pure cold-pressed castor oil. Speaking of gumming up, any engine that is run on castor oil should be cleaned very thoroughly prior to long term storage. I actually wash my engines twice - once in Varsol and then briefly for a second time in methyl hydrate. The latter step is mainly for the purpose of cutting down the "recently run diesel" aroma which some fellow residents of your home may find offensive! I then lubricate the engine with air tool oil, which I consider to be an excellent after-run oil - automatic transmission fluid and Marvel Mystery Oil are also very good. Even diesel engines treated in this way have no significant storage aroma and will remain nice and free for a very long time. The one positive thing to be said for castor gum if you do encounter it is that it is one of the most effective anti-corrosion coatings of them all - you'll never find a trace of rust under that 40-year old layer of goo! In discussing the properties of castor oil as a lubricant, once again we can do no better than paraphrase the comments of Bert Streigler in his previously-noted article. We saw earlier that an ideal lubricant should be "polar" enough to wet the moving surfaces – that is, it must have an affinity for the materials being lubricated. It should also have a high resistance to surface boiling and vaporization at the temperatures likely to be encountered. Finally, it should possess a large molecular structure to promote “oiliness”. Castor oil meets all of these rather simple requirements in a model engine context. It is highly polar, hence having a great affinity for metal surfaces, to which it adheres tenaciously. Roughly 87% of the make-up of castor oil is an organic compound called “triglyceride of ricinoleic acid”, which rejoices in the rather imposing chemical formula CH3(CH2)5CH(OH)CH2CH=CH(CH2)7COO)3(OC)3H5. A large enough molecule for you?!? It has a flash point of around 445 degrees F (229 degrees C). That's pretty hot in its own right, but the really amazing thing is that its fire point is way up at around 840 degrees F (449 degrees C) - an unusually large increase above the flash point. This means that castor oil will be very reluctant indeed to participate to a significant extent in the combustion process, even at elevated temperatures. By comparison, typical synthetic oils such as polyalkylene glycols flash at about 350-400 degrees F (176-204 degrees C). That's not all that far below the comparable figure for castor oil, but the bad news is that such oils typically have a fire point of only about 550 degrees F (288 degrees C) or slightly higher - way lower than the figure for castor oil. This means that castor oil is significantly more resistant to vaporizing at high temperatures than synthetic oil, also being far less prone to becoming involved in the combustion process. The one characteristic of castor oil which might initially appear as a drawback is the fact that it is thermally unstable. However, it turns out that it's this very instability that allows castor oil to continue to lubricate at temperatures well above those at which synthetics or mineral oils will generally break down. Triglyceride of ricinoleic acid is a unique compound in that its chemical formula includes a double bond in the 9th position and a hydroxyl in the 11th position. As the temperature goes up, it loses one molecule of water and becomes a "drying" oil which polymerizes readily. Castor oil has excellent storage stability at room temperatures provided it is stored in a well-sealed container in a cool, dark location. However, we've just seen that it polymerizes rapidly as the temperature goes up. As it polymerizes, it forms ever-heavier "oils" that are rich in esters and have excellent lubrication properties. These esters do not even begin to decompose until the temperature hits about 650 degrees F (343 deg C). Castor oil forms huge molecular structures at these elevated temperatures - in other words, as the temperature goes up, the castor oil exposed to these temperatures responds by transforming itself into an even better lubricant!
The downside is that this layer doesn’t go away – rather, it tends to build up over time, potentially tightening the fit of the piston in the bore. This can progress to the point where it requires removal to restore the engine’s dimensional stability. The one situation in which it can have a beneficial effect in this regard is with a well-worn engine – the varnish layer can actually help to maintain compression over and above what it would be without the varnish. This can become painfully apparent if you clean the varnish off the bore of a well-used engine (using fine soft-metal brushes and a solvent like Hoppe’s No. 9 Gun Cleaning Solution). An older engine cleaned in this way can suddenly start to feel a lot older yet – it was the varnish that was maintaining the required fits! The tendency of castor oil to form varnish can be significantly reduced through the use of a proportion of polyalkylene glycol synthetic oil such as Union Carbide's UCON or their MA 731. In fact, such a mixture can have some synergistic properties – in other words, better properties than either product had alone. However, care is required to establish the ideal proportions of the two oil types to achieve this desirable result. In extreme conditions, the castor content should always predominate. It’s interesting to note in passing that castor oil can be stabilized to a degree by the addition of Vitamin E (Tocopherol) in small quantities. However, if it is rendered too stable in this manner, it will lose the previously-discussed thermal responses that allow it to offer the unique high temperature protection that it does in its unadulterated state. Hence there seems to be little point in pursuing this approach. Castor oil is not normally soluble in ordinary petroleum fractions such as gasoline. However, if it is polymerized by heating it for several hours at 300 degrees F (149 deg C), the polymerized oil becomes soluble in such fractions. Hydrogenation of the oil achieves somewhat the same effect. Castrol R40 and Klotz Benol are examples of polymerized castor oils which are soluble in gasoline – they were widely used in two-stroke racing motorcycles when I was road-racing such machines.
Synthetic oils are man-made lubricants composed of organic and inorganic base stock oils combined with polymer packages to produce synthesized oil compounds. Depending on where you live, brands under which this type of oil is sold include Castrol, UCON, Pennzoil, Klotz, Dow, ML-70, PowerLube, F&B, G-Max, Amsoil, etc. Manufacturers normally term these products synthetic ester, polyglycolic or polyalkylene oils. A lot of present-day fliers use Coolpower Blue Synthetic oil. This oil mixes with both methanol and gasoline. I have no independent information regarding its relative lubricating qualities in a model engine application. I still add some extra castor oil to my Coolpower glow fuels! Once again, the well-informed insights of Bert Streigler are invaluable when discussing synthetic oils. On the positive side, such oils have excellent miscibility with almost all types of base fuels and are available in a wide range of different viscosities. They give rise to relatively little carbon deposition and do not form varnish on the cylinder walls. They also generate exhaust residues which are far easier to clean than those from operation on castor oil. Finally, they do not form gum deposits during long-term storage of an engine. However, there are some significant downsides which need to be carefully considered. To begin with, nearly all of the common synthetics that are used in model engines will burn in the combustion chamber if the engine is run too lean. Castor oil does not do this, both because of its very high fire point and because its response to elevated temperatures is to form more and more complex polymers with even better lubrication qualities. By contrast, most synthetics will boil on the cylinder walls at temperatures only slightly above their flash points. Once this occurs, the oil's lubrication qualities are greatly impaired. The same process can even occur in the wrist pin area, depending on engine design. The fact that synthetic oils do not polymerize at elevated temperatures or form varnish means of course that they do not offer the high temperature insurance that castor oil provides. The negative consequences of overheating and lean runs are thus likely to be far more drastic using synthetic oil than with castor. This appears to be a powerful argument in favor of using a mix of the two oil types, and indeed this is a common practise today among competition modellers. Like castor oil, synthetic oils are thermally unstable. However, unlike castor oil they do not polymerize into an even better lubricant - instead, they have a tendency to return to the basic materials from which they were made, usually things like ethylene oxide, complex alcohols, or other lubricants which are far less suitable for the purpose at hand. This process happens very rapidly once a critical temperature is reached. Lubrication engineers call this phenomenon "unzippering" for obvious reasons. Once this process is initiated, the lubricity of the oil is drastically reduced. Again, a mix of the two lubricant types appears to be indicated. The fact that synthetic oils have a marked tendency to become vaporized and participate in the combustion process generally results in higher cylinder temperatures when using synthetic oil compared to castor oil. More critically, oil that is vaporized and burned is no longer available to serve its lubrication function. This can leave the upper cylinder area somewhat oil-starved, to the point where a dry friction condition may be approached at elevated temperatures. Another result of this situation is that less residual oil is available to purge the engine of impurities and micro-particulates. These are not positive factors, especially the fact that synthetic oils are far less effective than castor oil as a high temperature lubricant. As a result, it’s important to manage engine temperatures very carefully when using synthetic oil. Reviewing all of the above information, it seems fair to say that if you intend to run your engine really hard, you should not rely upon synthetic oil unless you are willing to accept the increased wear and risk of damage that will inevitably result when compared to the use of castor oil. If on the other hand you plan to run the engine well within its limits (as in Scale, R/C sport flying or classic Old Time Stunt, for example), then you’re probably fine using synthetic oils if you want to do so. Ester acids can be formed during the combustion of synthetic oils. Since anti-corrosion agents are not added to all synthetic oils, it's wise to inject the engine with a little preservative oil after use. Preferably this should be done while the engine is still hot, allowing the injected oil to penetrate everywhere. I have found both air tool oil and automatic transmission fluid to be very effective after-run lubricants. When using synthetic oils, you should also be aware that the exhaust fumes produced when using such oils may cause allergic reactions or irritations. Specifically, exhaust gases may irritate the respiratory tract and eyes. Finally, an interesting potential consequence of switching from castor oil to synthetic oil should probably be mentioned here. Synthetic oils generally have quite pronounced cleansing qualities. In particular, they can dissolve any varnish build-up that may have accumulated within the engine while it was operated on castor oil. This may sound like a good thing, but it can be a real problem with a well-used motor which has still been performing well using castor oil. As the varnish layer is removed, such an engine can suddenly start to feel completely worn out! This is not a result of poor lubrication by the synthetic oil, but simply due to the removal of the varnish layer which has been helping to maintain the engine’s apparent working fits. Just something of which to be aware …………. Through the years, many modellers have tried various additives such as Wynn’s, Polyol B, Redex, 3 in 1, Lubricin N-1, molybdenum disulphide etc. Most of these have questionable efficacy in a model engine context, while some (such as Molyslip) can give rise to fuel system clogging and other problems. The only additive of which I am aware that has any real effect is Lubricin N-1 when used with a vegetable oil. This is a common ester which combines with a vegetable oil to maintain its lubrication qualities at reduced percentages of oil in the fuel. The use of this product makes it possible to reduce the amount of oil from 20% to 15% while retaining the fuel’s lubrication qualities. The result is higher power together with a cleaner engine. The amount used should be a maximum of 2-3% of the total amount of oil, otherwise there is a risk that the protective surface coating created by the castor oil could disappear, resulting in increased wear and higher operating temperatures. Testing of Oils
The results are shown graphically in the accompanying figures. The first figure shows the effect of changes in the fuel’s oil content on peak power output. As can be seen, power output increased with reduced oil content for both oils tested. However, the graph is silent on the issue of wear rates. A puzzling feature of this graph is the fact that it shows maximum power at 0% oil content, a condition which could not be When asked to comment on this graph, Maris Dislers pointed out that back when Luis conducted these tests it was fashionable to run really low oil contents in F2C team racing in order to maximize fuel economy. Daring people went for 5%, although most used around 8%. The engines involved would all be ABC or AAC. Maris agreed that there must be a practical sustainable minimum oil content for each combination of engine and oil type, whatever Luis’s power curve may show. I’m not plugged into today’s team racing scene, but Maris understands that current F2C team racing guys are using a bit more oil and are concentrating on maintaining an adequate castor oil ratio, with a proportion of synthetic oil/additive on top of the castor to keep carbon buildup under control.
It's worth mentioning in passing that mineral oils have the same effect on compression settings as castor oil provided temperatures are kept within limits, as they are in model diesels from the "pioneering" era. This is why oil content in the fuel is such a significant factor in the operation of fixed-compression model diesels, as discussed elsewhere on this web-site. By contrast, changes in the proportion of synthetic oil have very little effect, doubtless due to the tendency of this oil to vaporize and become part of the compressible fuel mixture in the combustion chamber. The oil thus becomes unavailable to reduce the effective volume of the combustion chamber. Of course, it also becomes unavailable to perform either its lubrication function in the upper cylinder or its purging function. This cannot be viewed positively in the context of engine wear rates. However, in a team racing context that appears to be of lesser concern.
By contrast, temperatures using castor oil peaked at around 20% and then fell off as the oil content was increased further. Reduced upper cylinder friction due to the elevated oil content doubtless had much to do with this. This figure incidentally constitutes strong evidence of the improved lubrication and reduced friction provided by higher ratios of castor oil in the fuel mix. It certainly explains why some engines (such as the PAW range and the old standby Fox 35) run much better and more consistently with elevated concentrations of castor oil in the region of 30%. Could it be that Duke Fox and Gig Eifflaender knew what they were talking about ....?!?
However, this graph throw up a bit of a surprise - the negative effect upon fuel economy (indicated here as a percentage of the ideal figure) is significantly greater using synthetic oil than it is using castor oil! To take just one example, the graph shows that it requires a 20% castor oil mix to reduce fuel economy to 95% of its optimum value, while only 12% of synthetic oil is required to produce the same negative impact upon fuel economy. This finding is so counter-intuitive that I wonder if the identification of the two curves has somehow been switched! The fact that the synthetic oil becomes in effect part of the power-producing make-up of the fuel (as evidenced by the temperature effects shown in Figure 3) should logically improve fuel economy by comparison with the non-combustible castor oil. However, assuming that the curves on the graph are correctly labelled, this does not appear to be the case. When I discussed this conundrum with Maris Dislers, he recalled Frank Coombs running tests with a PAW 149 in Mini Goodyear to achieve maximum fuel economy. It’s well known that PAW’s are particularly fond of a high castor oil content. Frank found that he could get superior economy by reducing the oil percentage, just as Luis suggests, but not for long. After a maximum of 20 minutes running on the lower oil fuel mix, the engine would no longer run consistently at the new leaner setting – it became prone to overheating and poor recovery from overheating. These negative effects took less time to develop as the oil content was further reduced. Frank postulated that the equilibrium that sustained the crucial castor oil “glaze” on piston/cylinder (or accumulated oil in the pores/ surface irregularities of the surfaces) had been lost due at least in part to its having been “washed off” by the other fuel components. This view was supported by the fact that a tank or two of oily fuel restored happy running. This scenario could be demonstrated repeatedly. Frank’s best successes were actually achieved using a fuel containing 30% castor oil, supporting my earlier comment on the benefits of higher castor oil ratios. Maris reports Frank as having recently surmised that in theory one could gain an advantage by using a low-oil fuel in a race or two with a well “oiled” engine, assuming one could quickly find the race tune on the day and get the job done before the accumulated protection was lost. Returning now to Luis’s tests, the one graph which is missing from these test results, obviously because of the impossibility of developing it, is one showing wear rates against oil contents. There can be no doubt at all that the type and quantity of oil must have a considerable effect upon this important factor. I can only report that experience in the field over a long period of time has demonstrated to my own satisfaction that castor oil is markedly superior to synthetic oil in terms of controlling wear rates – my castor-fed engines consistently outlast the identical synthetic-burning units of my colleagues. Reviewing the above information, I have to say that it appears to fully support my observations. I remain a die-hard castor oil man (except when running "classic" or fixed-compression diesels and old sparkers!). Conclusions
For all-out racing or in any situation in which the engine will be pushed, there’s simply no contest – use castor oil, or at least a good proportion of it! Overall, it's a far superior high temperature lubricant. However, for more laid-back flying where the engine won’t be pushed, by all means use synthetic oil if you want to. It may well be possible to achieve the best of both worlds by using an appropriate mixture of the two lubricant types. It’s impossible to draw any conclusion regarding wear rates from the above data – that is an issue which only long experience in the field can provide enlightenment. As stated earlier, my own experience over several decades of observation is that engines run on castor oil consistently outlast those of the same type operated on synthetic oil by a considerable margin. However, this is a purely empirical observation – I have no hard data to present. Only a sacrificial test using two carefully-measured brand-new identical engines which are run into the ground using the two types of oil could provide any meaningful data. I’m not volunteering- apart from the cost of the engines, think of the fuel bills!! That said, a number of firm conclusions can legitimately be drawn from the information presented in this article. These may be summarized as follows. The advantages of castor oil include the following:
The disadvantages of castor oil include the following:
The advantages of synthetic oil include the following:
The disadvantages of synthetic oil include the following:
In an F2C team racing context (in which Luis Petersen was writing), the main concerns are high power coupled with good fuel economy – engine wear rates are a secondary consideration. Luis’s test results show that oil mixtures of 20-30% castor oil and 15-25% synthetic oil are far too high for the development of maximum power allied to good fuel economy. In a typical F2C team racing application, Luis’s overall conclusion from this test was that an oil content in the fuel of 10%, made up of equal parts of castor oil and synthetic oil, provides the best compromise between power (5% increase), economy and operating temperature (3% increase). The likely price to be paid is a somewhat increased wear rate as well as less insurance against the effects of an overheating situation. Larger oil contents obviously provide a greater margin of safety for those who do not need to use the absolute maximum potential power output of their engines or wish to minimize wear rates. If you’re running an old classic that you wish to conserve, go heavy on the oil! In the end, the choice of oil comes down to a question of priorities. Castor oil undoubtedly offers superior insurance against the consequences of overheating and lean runs, also having superior film strength to deal with extreme pressures. However, the synthetics are still excellent lubricants as long as one recognizes their limitations and works within those limits. If a clean engine and model are important to you, that may be the way to go provided you’re confident of your ability to keep maximum temperatures under control. Used appropriately, engine life should be good with either product. However, cooked on a lean run, castor oil will win every time. An appropriate mix of the two can potentially offer the best of both worlds. Most glow-plug engines can get by with only a little castor oil in the oil mix, but diesels with their higher cooling loads and heavier wrist pin and crank pin pressures thrive on more castor oil in the mix. Like most things in this old life, lubricants are always a compromise of good and bad properties. We can and do get away with murder in our glow-plug engines because they are "alcohol-cooled" to a large degree. Diesels, though, can really stress the synthetics that are in general use today, hence doing far better with a generous amount of castor oil in the lubricant mix. I hope that the above article provides you with a basis for making an informed decision. If nothing else, both sides of this debate now have the facts at their fingertips. Good luck!! _______________________________________ Article © Adrian C. Duncan, Coquitlam, British Columbia, Canada First published February 2015
|
| |
 The issue of lubrication of model aero engines has always fascinated me. It’s a subject to which I’ve given considerable thought over the years along with a good deal of empirical observation. However, I’m by no means a qualified lubrication engineer. Consequently, my work in this field has never been of a truly scientific nature, always remaining within the limits imposed by the highly empirical nature of my personal observations.
The issue of lubrication of model aero engines has always fascinated me. It’s a subject to which I’ve given considerable thought over the years along with a good deal of empirical observation. However, I’m by no means a qualified lubrication engineer. Consequently, my work in this field has never been of a truly scientific nature, always remaining within the limits imposed by the highly empirical nature of my personal observations.
 However, this was not the only source of authoritative information on this topic. Following an outbreak of the oil wars in a well-known modelling magazine (well before the appearance of Luis’s article), a highly credible commentary was thrown into the debate by none other than the late Bert Streigler (of “Ebenezer” fame) on the subject of castor and synthetic oils. Although long retired at the time, Bert was the Senior Research Engineer at Conoco Oil Inc., hence being an individual who really knew his oils! There could be few individuals better qualified than Bert to comment upon this subject.
However, this was not the only source of authoritative information on this topic. Following an outbreak of the oil wars in a well-known modelling magazine (well before the appearance of Luis’s article), a highly credible commentary was thrown into the debate by none other than the late Bert Streigler (of “Ebenezer” fame) on the subject of castor and synthetic oils. Although long retired at the time, Bert was the Senior Research Engineer at Conoco Oil Inc., hence being an individual who really knew his oils! There could be few individuals better qualified than Bert to comment upon this subject. The development and application of lubricants suitable for model engine use paralleled the development of model engines themselves – both have advanced together over the years. The first model spark-ignition engines used gasoline as their basic fuel. The lubricant generally used in these engines was mineral oil, which blends very well with gasoline under all conditions. SAE 70 mineral oil was normally specified for this service, as it continues to be today. Although SAE 70 mineral oil is now very difficult to obtain, the later-generation mineral oils such as AeroShell 120 (SAE 60) are undoubtedly superior lubricants to anything that was available back in the '30's and '40's. Such oils can be used with confidence in old sparkies.
The development and application of lubricants suitable for model engine use paralleled the development of model engines themselves – both have advanced together over the years. The first model spark-ignition engines used gasoline as their basic fuel. The lubricant generally used in these engines was mineral oil, which blends very well with gasoline under all conditions. SAE 70 mineral oil was normally specified for this service, as it continues to be today. Although SAE 70 mineral oil is now very difficult to obtain, the later-generation mineral oils such as AeroShell 120 (SAE 60) are undoubtedly superior lubricants to anything that was available back in the '30's and '40's. Such oils can be used with confidence in old sparkies.
 Synthetic ester and polyglycol oils had their origins prior to the onset of WW2, but during that conflict they underwent intensive development for such applications as turbines and compressors. Following further development during the post-war era, such oils eventually began to find application as lubricants for model engines, a use to which they continue to be put today. In the beginning there were some serious problems with the use of such lubricants in model engines, but further development has resolved many of these problems to the point where today we have a legitimate choice of oil types to suit any given application.
Synthetic ester and polyglycol oils had their origins prior to the onset of WW2, but during that conflict they underwent intensive development for such applications as turbines and compressors. Following further development during the post-war era, such oils eventually began to find application as lubricants for model engines, a use to which they continue to be put today. In the beginning there were some serious problems with the use of such lubricants in model engines, but further development has resolved many of these problems to the point where today we have a legitimate choice of oil types to suit any given application.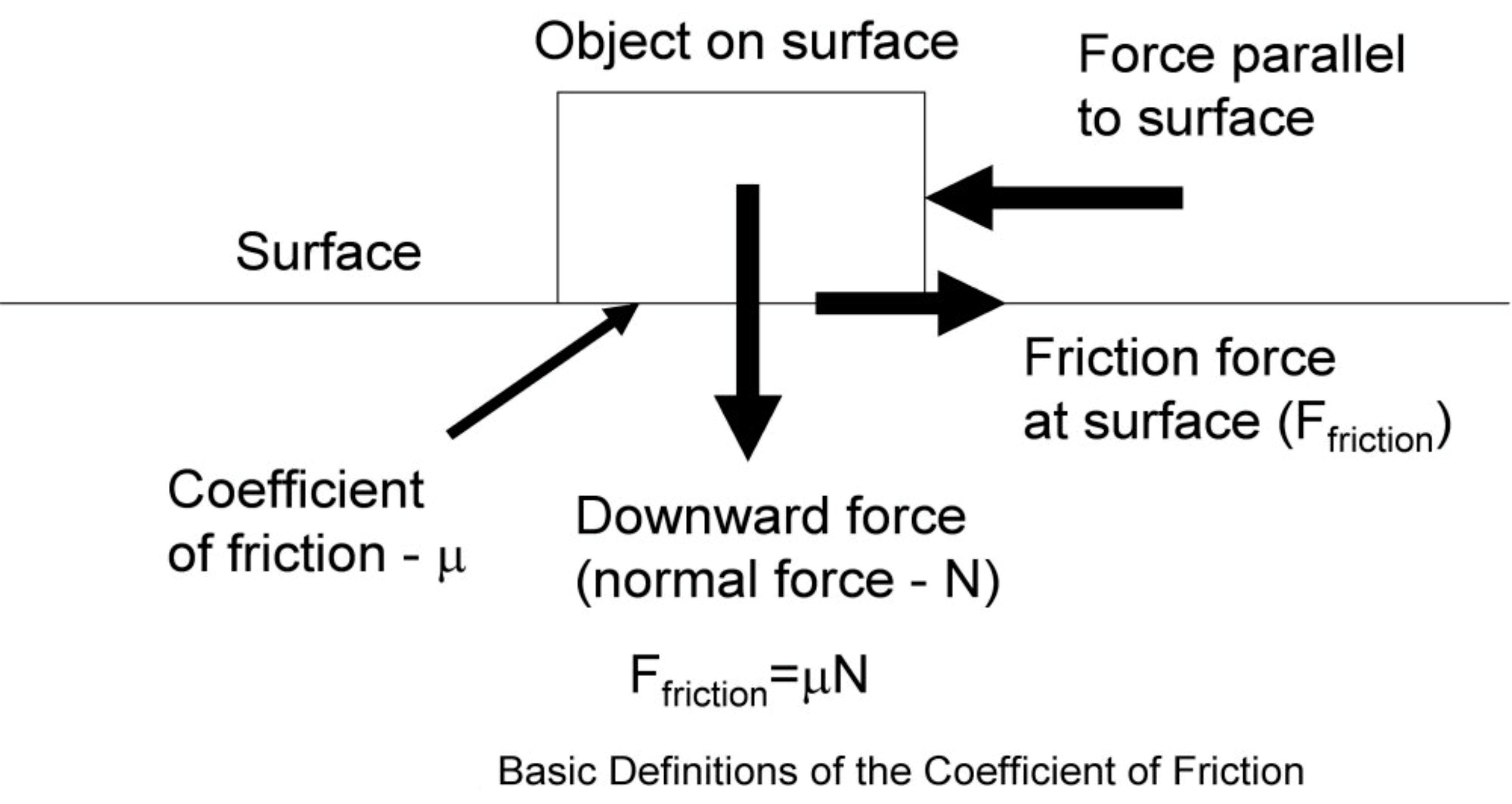 Friction can be divided into three categories, each of which has its own individual characteristics. Before discussing these categories, it’s important to understand the term used to quantify different levels of friction. For this purpose, we use a term call the “Coefficient of Friction”, generally assigned the symbol μ in technical discussions involving this parameter. For those not familiar with the term, the coefficient of friction is a dimensionless figure which describes the
Friction can be divided into three categories, each of which has its own individual characteristics. Before discussing these categories, it’s important to understand the term used to quantify different levels of friction. For this purpose, we use a term call the “Coefficient of Friction”, generally assigned the symbol μ in technical discussions involving this parameter. For those not familiar with the term, the coefficient of friction is a dimensionless figure which describes the 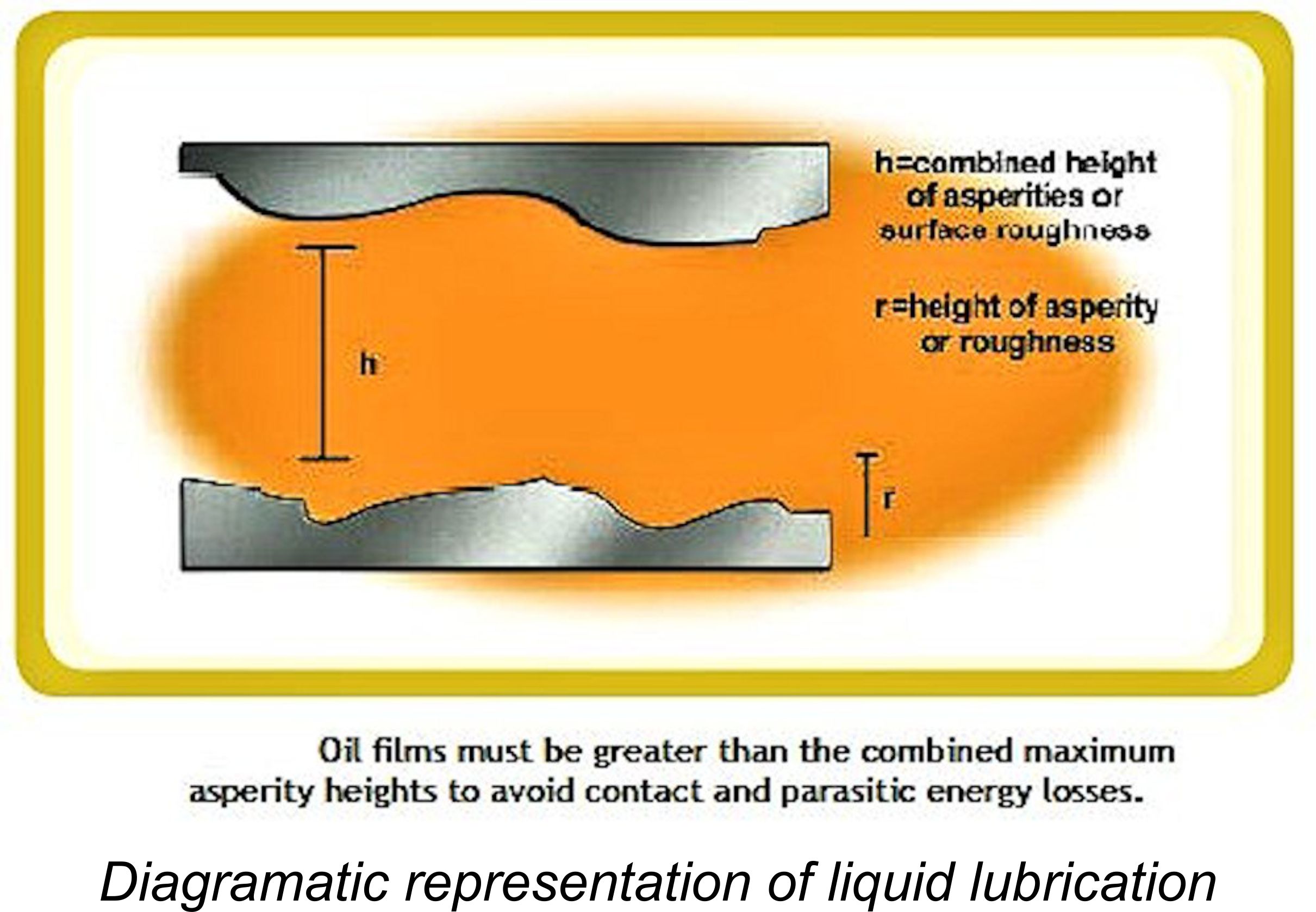 In this ideal condition, the sustainable lubricant film thickness is greater than the sum of the maximum heights of the surface irregularities (technically called asperities) present on both surfaces. Such asperities are present in all machined surfaces, no matter how finely finished. In this condition, even the peaks of the asperities on the two sliding surfaces are completely separated by a lubricant film, eliminating any contact between them.
In this ideal condition, the sustainable lubricant film thickness is greater than the sum of the maximum heights of the surface irregularities (technically called asperities) present on both surfaces. Such asperities are present in all machined surfaces, no matter how finely finished. In this condition, even the peaks of the asperities on the two sliding surfaces are completely separated by a lubricant film, eliminating any contact between them.  This value is an expression of an oil's resistance to flow. It is usually specified in centistokes (cSt). A full discussion of viscosity and its implications would go far beyond the scope of this very basic article, but suffice it to say that we are dealing here with a situation in which two materials are applying mutual pressure against one another and hence trying to make contact. The only thing that prevents contact from occurring (or minimizes the degree to which contact is made) is the lubricant film betwen the two surfaces. For contact to occur (the thing that we want to prevent), the oil film has to be squeezed out from between the two surfaces, and the only way in which it can do this is by flowing out through the very narrow gap between the surfaces. A fluid (oil) which is more resistant to flow will be more reluctant to flow out through a given gap between the surfaces, or will require a wider gap between the two surfaces through which to flow. Either way, the more viscous lubricant will be more effective in maintaining a gap between the two surfaces and hence promoting either boundary or liquid lubrication.
This value is an expression of an oil's resistance to flow. It is usually specified in centistokes (cSt). A full discussion of viscosity and its implications would go far beyond the scope of this very basic article, but suffice it to say that we are dealing here with a situation in which two materials are applying mutual pressure against one another and hence trying to make contact. The only thing that prevents contact from occurring (or minimizes the degree to which contact is made) is the lubricant film betwen the two surfaces. For contact to occur (the thing that we want to prevent), the oil film has to be squeezed out from between the two surfaces, and the only way in which it can do this is by flowing out through the very narrow gap between the surfaces. A fluid (oil) which is more resistant to flow will be more reluctant to flow out through a given gap between the surfaces, or will require a wider gap between the two surfaces through which to flow. Either way, the more viscous lubricant will be more effective in maintaining a gap between the two surfaces and hence promoting either boundary or liquid lubrication. 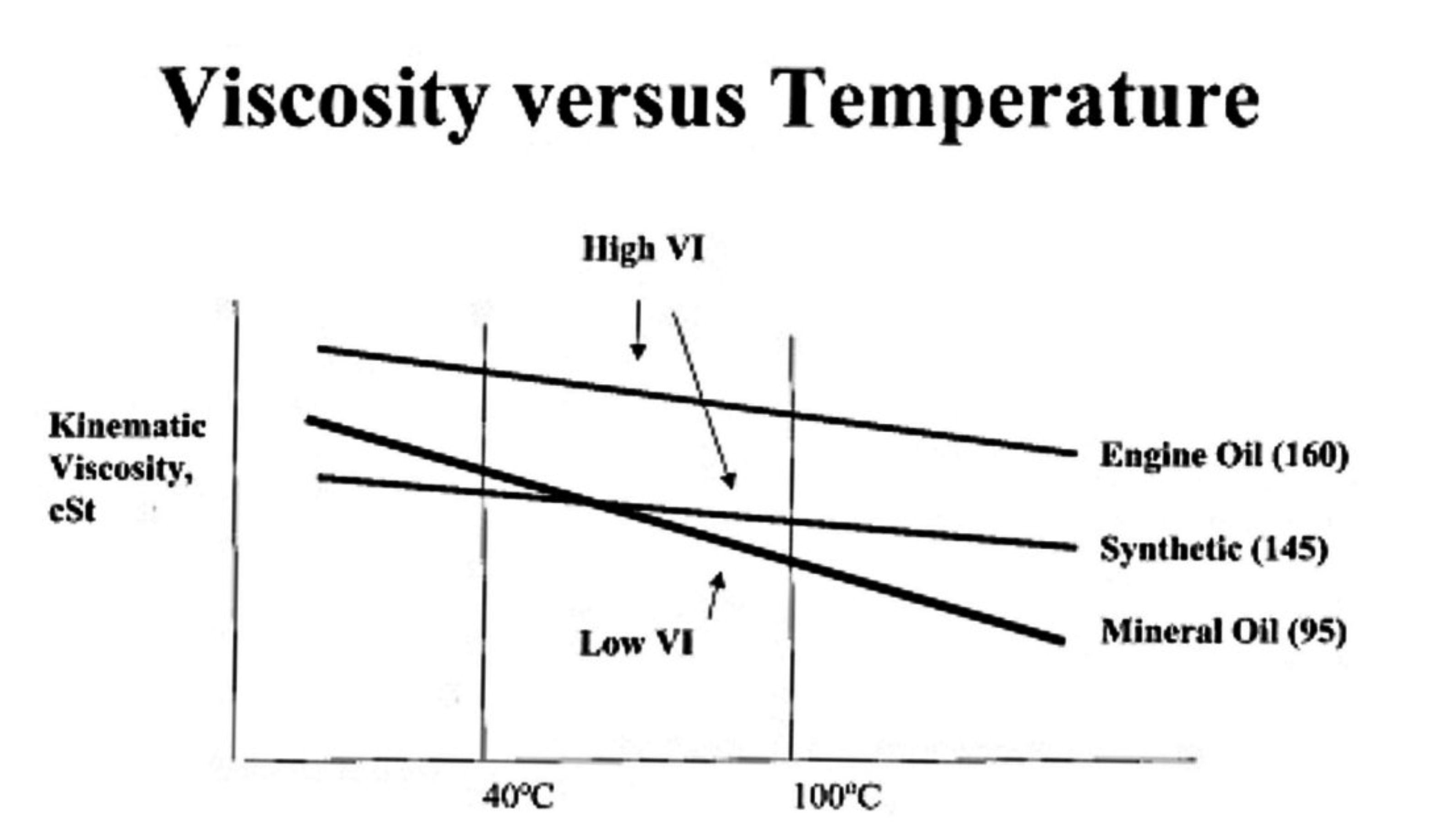 Viscosity Index
Viscosity Index Incompatibilities
Incompatibilities Mineral oil
Mineral oil castor oil, potentially vaporizing and burning quite readily in the temperature ranges which may be encountered in high-performance model engines. Hence they offer no insurance against such situations as lean runs or inadequate cooling. For this reason, they were generally replaced by castor oil in most model engine fuels quite soon after the conclusion of WW2.
castor oil, potentially vaporizing and burning quite readily in the temperature ranges which may be encountered in high-performance model engines. Hence they offer no insurance against such situations as lean runs or inadequate cooling. For this reason, they were generally replaced by castor oil in most model engine fuels quite soon after the conclusion of WW2. Vegetable oil
Vegetable oil source of castor oil, be aware that vegetable oils have a tendency to become rancid when stored for a long time. Always store such oils in a well-sealed container in a cool dark place. Stored in this way, they actually keep quite well.
source of castor oil, be aware that vegetable oils have a tendency to become rancid when stored for a long time. Always store such oils in a well-sealed container in a cool dark place. Stored in this way, they actually keep quite well.  Unfortunately, the ultimate by-product of this process is what we refer to as "varnish", which is an inevitable by-product of the use of castor oil in high-performance engines which operate consistently at elevated temperatures. However, even here there’s good news! The varnish layer itself is an excellent “dry” high-temperature lubricant, protecting the underlying metal surfaces very effectively. It bonds to the metal surfaces, fills the "valleys" between the asperities on the two surfaces and acts as a barrier to maintain a boundary lubrication condition. Hence it offers further insurance against the potentially ill effects of lean runs or overheating.
Unfortunately, the ultimate by-product of this process is what we refer to as "varnish", which is an inevitable by-product of the use of castor oil in high-performance engines which operate consistently at elevated temperatures. However, even here there’s good news! The varnish layer itself is an excellent “dry” high-temperature lubricant, protecting the underlying metal surfaces very effectively. It bonds to the metal surfaces, fills the "valleys" between the asperities on the two surfaces and acts as a barrier to maintain a boundary lubrication condition. Hence it offers further insurance against the potentially ill effects of lean runs or overheating. Synthetic oil
Synthetic oil Additives
Additives Some years ago now, Luis Petersen tested a model diesel engine using Castrol M (a typical castor oil) and MSSR (a typical synthetic oil) in various mixing ratios. The objective was to learn more about the impact of different oil types and differing oil percentages on model engines. These tests were conducted in the laboratory at Copenhagen Teknikum. A BG Bügl 15 team race engine was used as the test unit. The accompanying photograph shows the test set-up.
Some years ago now, Luis Petersen tested a model diesel engine using Castrol M (a typical castor oil) and MSSR (a typical synthetic oil) in various mixing ratios. The objective was to learn more about the impact of different oil types and differing oil percentages on model engines. These tests were conducted in the laboratory at Copenhagen Teknikum. A BG Bügl 15 team race engine was used as the test unit. The accompanying photograph shows the test set-up.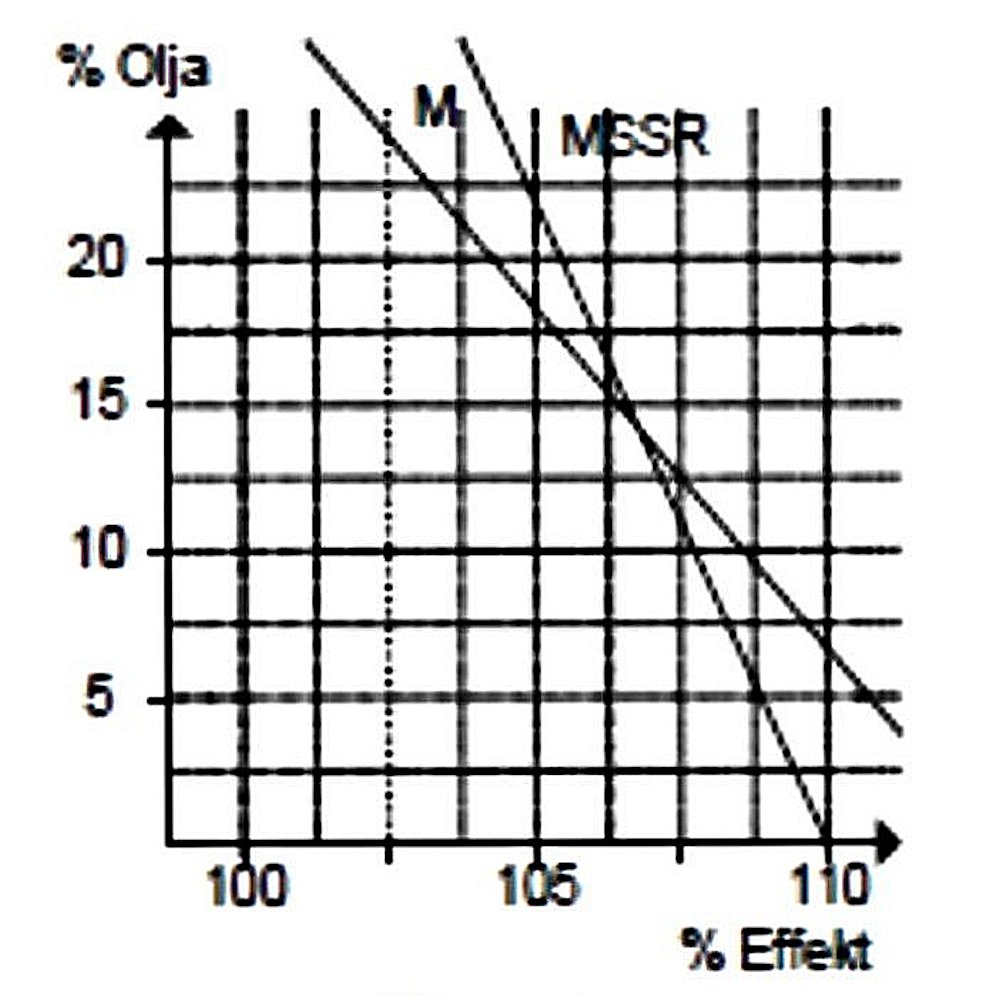 sustained for more than a few seconds without the engine seizing or at least sustaining very rapid wear. It’s very hard to believe that such an oil-less fuel mixture was actually tested! It is surely intuitively obvious that there must be a lower limit at which wear rates and friction losses become problematic.
sustained for more than a few seconds without the engine seizing or at least sustaining very rapid wear. It’s very hard to believe that such an oil-less fuel mixture was actually tested! It is surely intuitively obvious that there must be a lower limit at which wear rates and friction losses become problematic.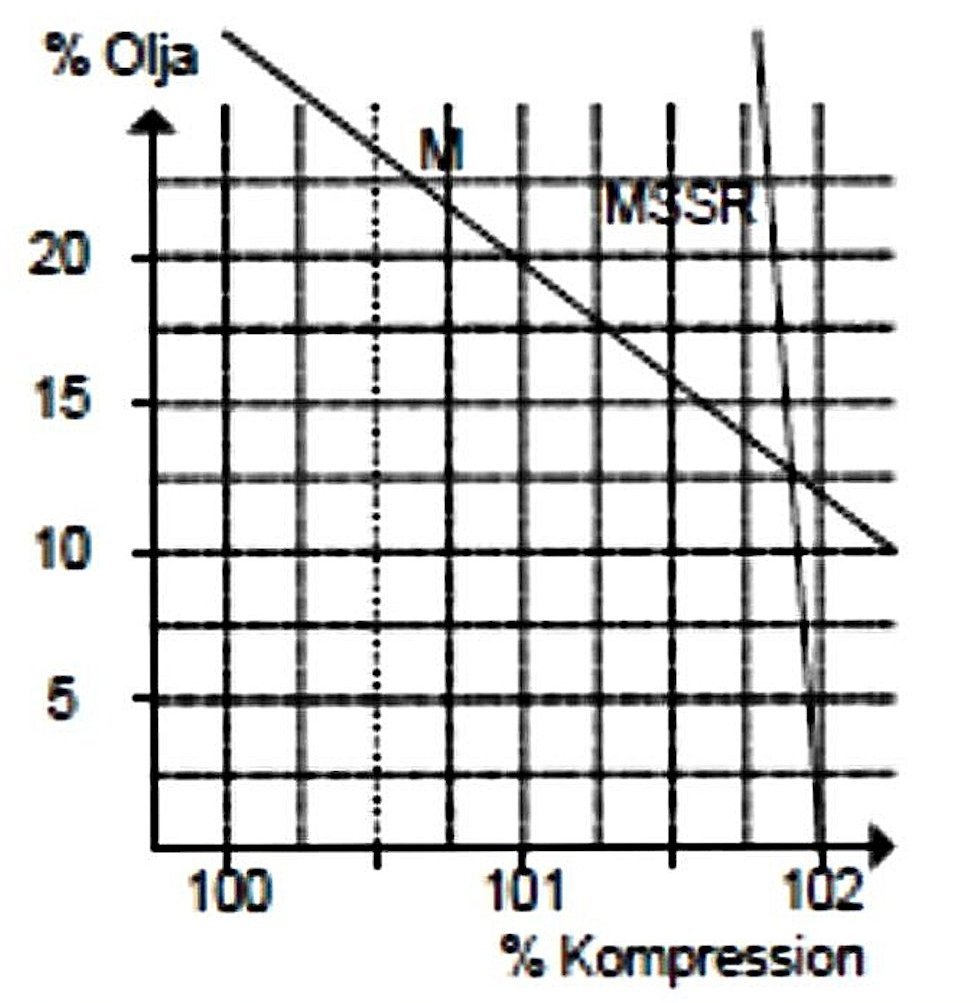 The next figure shows the effect of changes in oil content on the compression setting required to run the engine efficiently. As can be seen, the effect of adding more castor oil is to reduce the compression requirements, although the effect is relatively small. This is because the oil which condenses out of the fuel mixture takes up space in the combustion chamber, which in turn reduces the effective volume of the combustion chamber, hence increasing the working compression ratio above that at which it is set in purely geometric terms.
The next figure shows the effect of changes in oil content on the compression setting required to run the engine efficiently. As can be seen, the effect of adding more castor oil is to reduce the compression requirements, although the effect is relatively small. This is because the oil which condenses out of the fuel mixture takes up space in the combustion chamber, which in turn reduces the effective volume of the combustion chamber, hence increasing the working compression ratio above that at which it is set in purely geometric terms.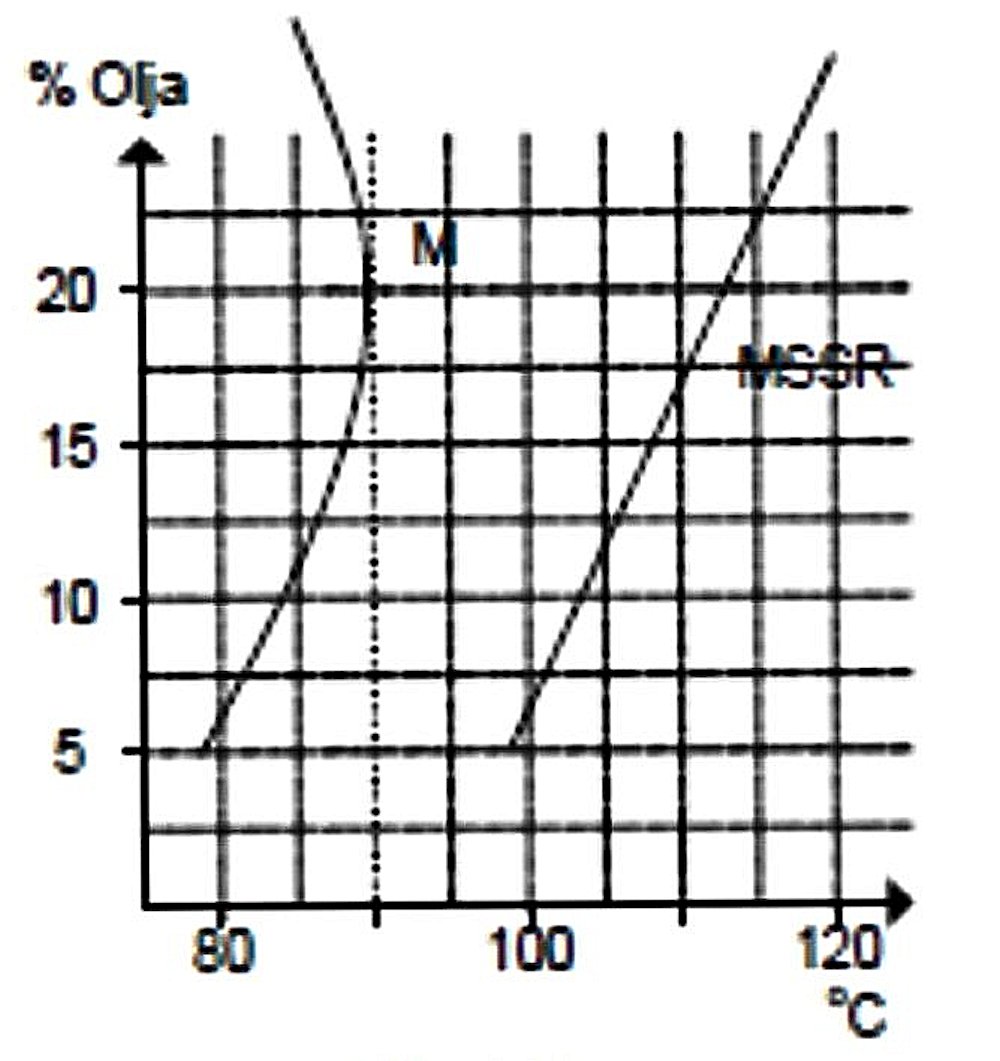 The next figure shows the effect of changing oil ratios upon cylinder head temperatures. As can be seen, the engine ran considerably hotter at all mixing ratios using the synthetic oil than it did using castor oil. This is doubtless due in large part to the synthetic oil becoming involved in the combustion process along with the base fuel. As we would expect, temperatures continued to rise as the proportion of synthetic oil was increased, since the effect of this was simply to add more high-energy fuel to the mixture. This is about as convincing a demonstration as you could want that at least some of the oil is participating in the combustion process.
The next figure shows the effect of changing oil ratios upon cylinder head temperatures. As can be seen, the engine ran considerably hotter at all mixing ratios using the synthetic oil than it did using castor oil. This is doubtless due in large part to the synthetic oil becoming involved in the combustion process along with the base fuel. As we would expect, temperatures continued to rise as the proportion of synthetic oil was increased, since the effect of this was simply to add more high-energy fuel to the mixture. This is about as convincing a demonstration as you could want that at least some of the oil is participating in the combustion process. 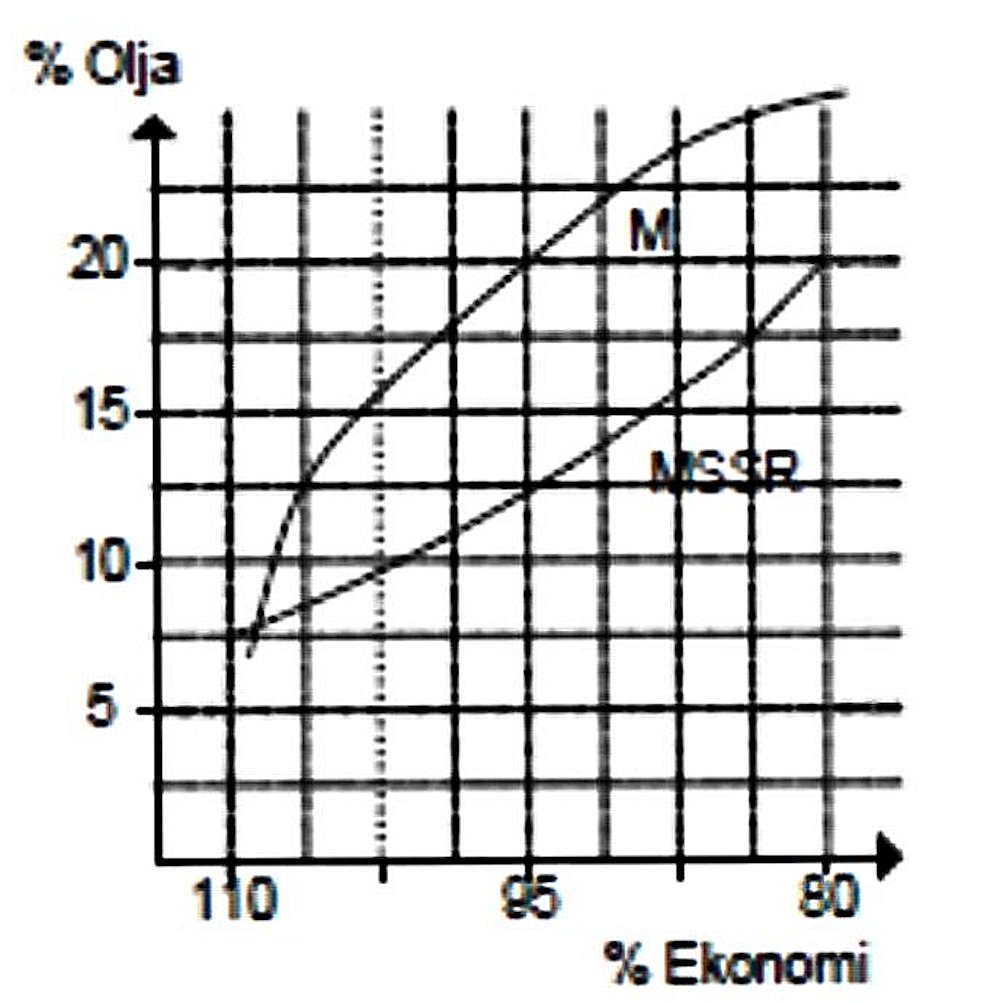 The final figure shows the effect of changes in oil content upon fuel economy. We would of course expect that higher oil percentages would have an adverse effect upon fuel economy, since the theoretically-incombustible oil takes up volume in the fuel tank which is then unavailable for the power-producing constituents of the fuel, requiring the needle to be opened to compensate. And indeed, that is what we see for both synthetic and castor oils.
The final figure shows the effect of changes in oil content upon fuel economy. We would of course expect that higher oil percentages would have an adverse effect upon fuel economy, since the theoretically-incombustible oil takes up volume in the fuel tank which is then unavailable for the power-producing constituents of the fuel, requiring the needle to be opened to compensate. And indeed, that is what we see for both synthetic and castor oils.  The choice between castor oil and synthetic oil is not a cut-and-dried matter. Depending on the application to which a given engine will be put, I hope I've made it clear that both sides can stand on some pretty high ground. This choice has to be made largely on the basis of how one intends to use his engine – all-out racing or Sunday sport-flying.
The choice between castor oil and synthetic oil is not a cut-and-dried matter. Depending on the application to which a given engine will be put, I hope I've made it clear that both sides can stand on some pretty high ground. This choice has to be made largely on the basis of how one intends to use his engine – all-out racing or Sunday sport-flying.