
|
|
Cleaning and Preservation of Magnesium Alloy
With a density of around 1.8 gm/cm3, magnesium is the lightest structural metal in existence by some margin. It is also quite readily available, being in fact the 8th most abundant element in the earth's crust as well as the third most plentiful element occurring in solution in seawater. It occurs naturally in minerals such as Dolomite, Magnesite and Carnallite as well as in sea water and other naturally-occurring brines. Magnesium is produced from sea water, natural brines and magnesium-bearing minerals which together offer almost unlimited global reserves. 940,000 metric tons of the metal were produced worldwide in 2023. Magnesium alloys are mixtures of magnesium with other metals, typically aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Practically all the commercial magnesium alloys produced in the United States today contain aluminium (3 to 13 per cent), manganese (0.1 to 0.4 per cent) and zinc (0.5 to 3 per cent). At first sight, the benefits of using magnesium alloy to cast such components as model engine crankcases appear compelling. It's 75% lighter than steel and 33% lighter than aluminum. It has adequate structural strength allied to good stiffness, also displaying a high degree of dimensional stability. In production terms, it possesses a very favourable combination of good casting qualities with outstanding machining properties. Looking at the above summary, a model engine crankcase is a made-to-order application for such an alloy – at least, so it appeared to quite a few manufacturers of model engines during the classic era. The names of Arden, Bantam, Mite (USA), Zeiss, Kemp, Mills and E.D. come immediately to mind, and there are many others. However, there was a very large fly in this particular ointment! The highly reactive nature of magnesium means that if left to their own devices magnesium alloys have a greater-than-usual tendency to want to return to their original mineral state through a process of corrosion. In particular, magnesium has a great affinity for oxygen which renders it highly susceptible to oxidation. Water is a major contributor to such a reaction, which can take the form of either direct oxidation following the chemical formula Mg + H2O (water) = MgO (magnesium oxide) + H2 (hydrogen) or conversion to the hydroxide form following the chemical formula Mg + 2H2O = Mg(OH)2 (magnesium hydroxide) + H2. Both of these reactions involve the transference of oxygen to the magnesium from the water. The affinity of magnesium for oxygen is in fact so great that it is actually inflammable if heated sufficiently, combining directly with oxygen in the air to form magnesium oxide in the form of a white powder. During my early years of involvement with cave exploration, we often used magnesium ribbon to illuminate large underground chambers - it burns with an intense white light. The potential for magnesium shavings to ignite during machining operations is a recognized hazard, although it is perfectly manageable with due care. It was in fact the marked tendency of magnesium castings to develop corrosion over time that led to their general abandonment by the model engine manufacturing industry as of the mid 1960's. Broadly speaking, this corrosion can take two forms – galvanic corrosion and atmospheric corrosion. Let’s look at these two mechanisms in turn. Galvanic corrosion is an electrolytic ion-exchange process which takes place when a dissimilar metal is brought into direct contact with a magnesium surface in the presence of an electrolyte (such as salt water or oil which is contaminated with combustion residuals). Perhaps the most effective way to combat galvanic corrosion of magnesium is to prevent the required contact conditions from arising in the first place. However, this is a design issue about which little can be done after the fact. Prevention of this type of corrosion typically involves the use of isolation materials which are interposed so as to prevent direct contact between bare magnesium and the dissimilar metal. In present-day automotive practise, the use of appropriately-formulated aluminum washers can significantly reduce galvanic corrosion of magnesium alloy. However, the effectiveness of such corrosion protection is directly related to the chemical composition and consequent galvanic potential of the aluminum washer being used. Of course, in the construction of a model engine there’s pretty much no way of avoiding the dissimilar-metals scenario – at some locations steel components such as the cylinder, ball-race outer housings and assembly screws will make direct contact, as will such closely-fitted components as bronze plain bearing sleeves. To prevent galvanic corrosion in such situations, we need to exclude any possibility of an electrolyte being present in the first place. This form of corrosion also explains why it's essential to avoid any contact with steel or other metal abrasive tools such as wire brushes when cleaning magnesium castings. The use of such tools during cleaning sets up perfect conditions for the development of galvanic corrosion due to the inevitable microscopic "streaking" of the bristle material onto the magnesium surface. Atmospheric corrosion takes place when the unprotected surface of a magnesium component comes into contact with air having a measurable moisture content which can act in conjunction with the oxygen content of the air, some of which dissolves in water. Apart from containing some dissolved oxygen, moisture in air is slightly acidic as a result of the absorption of carbon dioxide to form carbonic acid, thus possessing electrolytic properties which encourage galvanic corrosion. Moreover, this acidity is rising at the present time as levels of carbon dioxide in the atmosphere continue to increase. The problem is intensified by the fact that impurities in the magnesium alloy set up what is in effect a microscopic but nonethless damaging galvanic oxidation situation which only needs the presence of an electrolyte (water) to get started. The result once again is that unsightly gray or white powdery deposit which mars the appearance of your engine. During the 1940’s and 1950’s when most of the engines with which we are concerned were manufactured, magnesium casting alloys were far less pure than they are now, hence being quite prone to atmospheric corrosion of this type. With the present-day high-purity alloys, the potential for loss of material to atmospheric oxidation is greatly reduced. This has allowed the ongoing use of magnesium alloys in the automotive industry, where they are routinely and increasingly employed with great success. But that’s now – we’re talking about then! We have to recall the fact that as far as I'm aware the era of magnesium-case model engines ended in 1964 with the final withdrawal of the Mills diesels. Consequently, any magnesium-case model engine which may be encountered today is over half-a-century old! As stated earlier, the alloys used in the ‘40’s and ‘50’s were less pure than they are today, hence being wide open to the atmospheric oxidation process. This means that any magnesium-case model engine from the classic era that is improperly stored and simply left to its own devices is doomed to turn eventually into a heap of gray/white powder as time goes by. We certainly don’t want that – something Of course, the manufacturers of the classic model engines with which we are concerned were well aware of the corrosion issue. A popular response was the use of a chromate surface treatment, as seen on the second-generation Mills engines among others. However, this treatment actually does more to prepare the surface for finishing with other materials such as paint than it does for corrosion protection. Moreover, it tends to wear thin or vanish completely with time, exposing the underlying metal to the full ravages of corrosion. In any case, the chromate-type treatments formerly used have since lost favour because of safety and environmental concerns. So we seem to be down to our last lines of defence, which are either to isolate the engine from any contact with the surrounding atmosphere (see further discussion below) or to apply some form of renewable protective coating to the magnesium which doesn’t affect the engine’s underlying form or finish but does prevent contact between the magnesium and either the surrounding air or any form of electrolyte. Since it's the most commonly-used protective measure, we'll begin by looking at the second of these two options. The ideal protective material would appear at first sight to be a penetrating lubricant which has moisture-displacing qualities. For many years now, I myself have used air tool oil as my basic protection material, also finding it to be an excellent after-run lubricant for any engine, mag case or otherwise. This highly penetrative lubricant is specifically designed to prevent corrosion in pneumatic systems in which air-borne I have always reckoned that these characteristics should make air tool oil ideal for use as a magnesium case protection coating, since the crucial thing is to displace any water that might otherwise come into contact with the metal and act as an electrolyte. That’s exactly what air tool oil is designed to do! It's also specifically intended to resist the development of oxidation conditions. You may hear recommendations regarding the use of WD-40 as a preservative. In fact, this material is useless for long-term preservation purposes. It's primarily intended for short-term use as a water-displacing cleaning medium, for which purpose it is quite effective. However, it works in large part by having hygroscopic (water-attracting) properties which allow it to absorb and carry off any water in the object or mechanism being cleaned. This means that if it is used as a long-term preservative, it will actually attract water to the surface over time - the reverse of what we want! In this way, it contributes to the development of further corrosion. Moreover, its lubricant properties are very short-term, degrading very rapidly over time. Hence it won't keep your engine lubricated while in long-term storage. My advice - don't use it! Overall, I've found that WD-40 is a somewhat over-rated product in general. It's widely viewed as a magic do-anything elixir, but in fact its real utility is confined to acting as an initial cleansing medium, where its water-displacement qualities (hence the name WD) may indeed be useful. However, that's about it. Check the Web and judge for yourself ............... Cleaning Of course, before you have an engine worth preserving you have to clean it - those unsightly patches of grey or white corrosion residues have to go! However, I should add a word of warning at this point to prospective purchasers of corroded mag case motors - cleaning them will not restore any lost material. On the contrary, any magnesium that has been converted to corrosion residues will be permanently removed by the cleaning process. The surface will accordingly have lost some metal which will manifest itself in the form of pitting or etching. Something to be aware of when considering the purchase of a fairly well-corroded motor.
However, that's just me! To see what other techniques might be lurking out there, my valued friend Jerry Turnbaugh of Pasco, Washington, USA kindly undertook a Web survey of potential approaches to cleaning magnesium alloy castings. Jerry hastened to note that he had not tried cleaning or preserving magnesium alloys himself. Consequently, he couldn't personally vouch for the effectiveness or suitability of the techniques and chemicals discussed. However, he was knowledgeable regarding these techniques and chemicals in general, having used many of them on other metals. This being the case, Jerry pointed out that as with any untried process, these techniques and chemicals should be tested on an inconspicuous portion of the item to be cleaned, or a representative sample, before committing to full-scale use. These techniques and chemicals can be used separately or in combination. There is a huge variety of chemical cleaning agents on the commercial market. For example, if you want a "de-greaser", a trip to a paint shop, hardware store or automobile parts outlet will quickly overwhelm you with the variety of de-greasers to choose from, as will a search on the Internet. For this reason, Jerry tried to stay with common household and chemical names with which most hobbyists are familiar, merely noting their general categories. It's up to you which brand(s) you choose to employ. The basic strategy is to first remove existing corrosion and then prevent future corrosion by keeping the clean magnesium alloy surface away from contact with water in any form. Jerry correctly noteed that a far better job of cleaning and protecting a complex shape like a model engine casting can be achieved by disassembling the engine so that the magnesium alloy parts can be handled separately. This is particularly important when chemical cleaning agents (as opposed to lubricants) are to be used, since they can potentially attack the other metallic components.
As a preliminary, the part should be de-greased. Automotive carburettor cleaner or isopropyl (rubbing) alcohol are two readily available candidate media for this purpose. It may be wise at this stage to plug the shaft bearing with a wooden plug or something else to keep residues and cleaning solutions away from the bearing, which is very likely some material other than magnesium alloy. There's a very wide range of techniques that can be used to actually remove any corrosion residues. However, there are a few basic rules. First, the use of abrasives such as fine sandpaper, Scotch Brite or the like should be strenuously avoided. While such methods would quickly remove any corrosion, they would inevitably leave micro-scratches which would both mar the surface finish of the component and serve as prime sites for the onset of further corrosion in the future. Another no-no is the use of even the finest metallic brushes such as those made of steel or brass. This is because (as mentioned earlier) these tools inevitably leave "smears" of metal on the surface being cleaned, which will form focal points for the onset of galvanic corrosion in the future. A useful tool for digging out or scraping off bits of dirt, hardened oil residues and the like can be made by taking a standard 1/8 in. dia. bamboo skewer of the type used for fondue cooking and sand a chisel point onto one end. This will deal with most deposits requiring physical removal without marring the metal surface. Other non-metallic cleaning tools such as Cratex wheels or non-metallic brushes have their supporters on the Web and may be appropriate if used carefully. My own use of old nylon-bristle toothbrushes seems to square well with this recommendation. Jerry noted a comment by Greg Holland which appeared in the "Vintage Slot Racing Newsletter" to the effect that bead blasting appeared to be the most effective cleaning method for corroded magnesium alloy castings, based on a number of tests on various techniques. However, this may be further than most model engine owners are able or willing to go.
Several formulae for making a home-brewed alkaline cleaner can be found on the Web, mostly using sodium hydroxide (NaOH, often sold as a drain cleaner under the name lye), TSP and sodium carbonate (Na2CO3). Here are a couple of such blends which Jerry found on the Web, with their recommended application parameters: 1) In one litre of water, dissolve 50 gm of NaOH (lye) and 10 gm of TSP. Immerse part for 6-10 minutes at 55 - 65 degrees Celcius. 2) In one litre of water, dissolve 30 gm of Na2CO3 and 30 gm of TSP. Add 1 gm of dish detergent (not dishwasher detergent!). Immerse part for 3-20 minutes at 80-95 degrees celcius. We haven't tried either of these formulae, so you're on your own if you wish to do so!! For my part, I think I'll stick to the toothbrush and oil approach unless I get a really bad component! Still, sincere thanks to Jerry for researching this topic for us so thoroughly. We'll hear from him again further down the page. Preservation OK, so one way or another we now have a nicely cleaned mag-case motor on our hands. How to keep it that way?!? Once again, I'll begin with my own approach, which has so far appeared to work pretty well. Handling the cleaned engine while wearing thin vinyl gloves to prevent any skin contact and consequent moisture introduction, I inject it through the intake with a healthy dose of air tool oil which is then swished about liberally inside the engine by turning it over and over with the oil inside. I then take an artist's brush of suitable size and “paint” the magnesium surfaces all over externally with air tool oil, paying particular attention to the nooks and crannies. After this, I wrap the oil-coated engine quite tightly in a dry plastic baggie to minimize the opportunity for circulating air or any other moisture source to come into contact with the engine. I then store it in the driest place that I can find in the house. From time to time I inspect all my mag-case motors and repeat the “painting” process. The procedure is So far, this seems to have worked very well for me – some of my mag-case motors go way back in my care! However, I don’t claim to know it all – in fact, I’ve reached that happy state of wisdom in which I finally know enough to fully appreciate how little I know! For this reason, when considering the preparation of this article I approached a few others to find out what they recommend. My good mate Maris Dislers replied that his approach is pretty much the same as mine except that he uses gun oil rather than air tool oil. He stores the oiled engines in a plastic container or bag, just as I do. He also mentioned having a new E.D. Racer magnesium crankcase as a spare part. This must have been manufactured at least sixty years ago, but it remains in perfect condition. It's apparently covered with some kind of factory-applied wax or similar coating to protect it. The coating would presumably come off with a solvent wash prior to installation. It seems that wax dipping was a recognized approach to the preservation of individual magnesium castings. However, it would not be a practicable approach for complete engines.
This sounds like a very promising material to use - too bad that it doesn't seem to be readily available here in Canada. It is however available in the USA. North American residents can email INOX's US distributor Justin McCartney at jmccartney904@outlook.com to check on availability in their area. He responded very promptly to my own inquiry. Tandy Walker recommended the use of Tri-Flow oil for preserving magnesium castings. This is apparently used by locksmiths to lubricate the internal workings of precision locks, but it works well for our purposes also. It seems to be readily available. In passing, I'd like to point out that it's necessary to be quite careful when selecting materials of this kind. Always read those MSDS documents!! As an example, when reviewing similar products available locally, I ran across one that is sold by the 3M Corporation under the name 3M (TM) 5-Way Penetrant. This is a multi-purpose product that is reportedly such an effective moisture displacement agent that it can be used to dry electrical apparatus. It also contains a lubricant fraction. It is apparently effective as a penetrating oil for freeing corroded bolts and the like, and is also promoted as being an effective surface cleaner. Sounds perfect............ until you read the MSDS sheet, which classifies it as extremely hazardous to your health! I'll stick with air tool oil unless I can get my hands on some INOX MX3! Next up was my greatly-valued friend and colleague Kevin Richards, who is undoubtedly the world's leading authority on E.D. engines. As such, Kevin has a fair number of mag cases to preserve! His first piece of advice is - keep magnesium cases well away from long-term contact with cardboard boxes! The cardboard is slightly acidic, hence going to work quite rapidly on any magnesium with which it comes into contact. If you must store a mag-case engine in its box (or in a larger box along with others), make sure that it's very well wrapped in an intact plastic baggie. I generally store my mag-case engines and boxes separately.
Kevin may well be on to something here. Vaseline is basically petroleum jelly, which has long been known to be useful in coating corrosion-prone items such as metallic trinkets, non-stainless steel blades and gun barrels prior to storage. Its effectiveness seems to be down to the fact that it has extremely good water-repellant properties. It is actually used as an environmentally friendly underwater antifouling coating for motor boats and sailing yachts. It has even been recommended in the Porsche owner’s manual as a preservative for light alloy wheels to protect them against corrosion from road salts and brake dust. Porsche recommends that "every three months (after regular cleaning) the wheels should be coated with petroleum jelly.” If it's good enough for Porsche .............. Kevin also noted that it is possible to re-chromate magnesium cases. However, this is an extremely toxic and environmentally-harmful process which is definitely best avoided in an amateur's workshop. Finally, Jerry Turnbaugh put a considerable amount of on-line research into this aspect of the matter as well. He agreed wholeheartedly with Kevin Richards' recommendation that chromate treatment be avoided, and for the same reasons. Jerry's next point was that once the magnesium alloy part has been thoroughly cleaned, great care must be taken to avoid re-contamination with skin oils, sweat or similar. This is why wearing vinyl gloves while handling these engines is a good idea. The basic requirement is to prevent any contact with liquid water or atmospheric moisture. This is particularly critical in areas where high humidity levels are combined with proximity to salt water. I live in just such an area myself. In an ideal situation, one would isolate the engine from the surrounding atmosphere by storing it in a sealed air-tight jar with dry silica-gel dessicant. The absolutely optimal approach would be to store the engine in a disassembled state so that galvanic corrosion could not occur under any circumstances. However, that is not a practicable approach for the average engine aficionado - what would be the point?!? Still, keeping a complete engine in a sealed container with a dessicant supply does seem to have some potential. Big thanks to Jerry for putting this suggestion forward! Another approach which Jerry noted is to store the engine in a vacuum sealed bag (a common approach to food preservation). This would minimize the potential amount of moisture in contact with the engine, although the vacuum would invite the invasion of moisture over time due to the pressure differential between the interior of the bag and the surrounding atmosphere. Jerry saw considerable merit in Kevin Richards' suggestion of the use of petroleum jelly as a preservative coating. He cited experience during WW2 with Cosmoline, which is a substance somewhat similar in nature to Vaseline. It has been used very successfully as a corrosion-resistant surface treatment for military equipment. In fact, Cosmoline remains available today, being highly recommended as a preservative for firearms and other precision metallic articles. If you can find a source, it may well be worth a try. Finally, there are of course a variety of "clear coats" that one could theoretically use following cleaning. However, these would very likely not be fuel-proof, and in any case would alter the appearance of the engine. Similarly, one can find a number of silicone-based "waterproofing" sprays which are readily available. However, the manufacturer of one common variety which Jerry ran across claims that their waterproofing sprays keep water out but allows water vapor to "breathe" through it! Huh?!? Doesn't sound too good for magnesium alloy!! Following the original publication of this article, I heard from Alan Strutt, who made me aware of another widely-recognized approach to the care of magnesium castings. Alan had used this approach himself, thus being well-placed to describe it for us. Alan noted that the recommended approach to the treatment of magnesium castings in full-sized aircraft service is the application of a chemical compound called selenous acid. In May of 2002 Alan obtained some 99% pure selenous acid in crystal form. However, he pointed out that this compound is a key ingredient in gun bluing and brass antiquing solutions. This may be a more convenient way to obtain a supply if the chemical concerned does indeed contain selenous acid. Alan tried this approach on two engines - a Millls 2.4 diesel and a Microdyne Atom Mk. 1, both of which were suffering from the typical white powder corrosion brought about by the damp English climate. After cleaning the engines externally, he made up a solution of the selenous acid and applied it to the surfaces using an artist's brush. All traces of the white corrosion products quickly disappeared with repeated applications. The only observable issue was a slight reddening of the surface, particularly where the corrosion residue had been formerly. After allowing the cases to dry thoroughly, Alan oiled the engines internally with gun oil and then stored them in Ziploc freezer bags with no external oil application. A small silica gel pack is stored with each bagged engine, and Alan warmed these up periodically to dispell any accumulated moisture. The engines had been stored in this way for thirteen years prior to Alan's communication with me, with only a few very small traces of recurring corrosion on the Mills and the Atom remaining in perfect condition. This without any external oiling whatsoever. Alan really liked the fact that he could handle these engines without getting his fingers covered with oil or Vaseline! Alan commented that the chemistry involved in this application is somewhat unclear. He felt it to be likely that the acid is reacting with the alkaline earth metal (magnesium) to form either an elemental coating of selenium or magnesium selenite. It appears that either or both of these chemicals passivate the underlying magnesium against further electrolytic or moisture-associated corrosion. I actually tried gun blue myself some years ago (knowing nothing of the chemistry involved), finding that while it did indeed remove the corrosion, it also changed the surface color somewhat. It was for that reason that I didn't mention it in the original text. I should go back and take another look at this approach ............. If you plan to do the same, please take the time to acquaint yourself with the appropriate precautions to take when using such materials. I'd also strongly advise that you try a test piece first to assess the effect (if any) upon the surface finish. Conclusion
When it comes to the cleaning stage, Jerry Turnbaugh summarized a number of possible approaches that have been documented on the Web, while others (including myself) have shared the techniques which have been found to be effective over the years. A number of the techniques and materials summarized by Jerry remain untested by us. Accordingly, anyone wishing to try any of them is strongly advised to test them first on a non-critical area of the casting (or better yet, try them on a scrap casting). As far as preservation of mag-case model engines goes, it appears that the use of a water-repellent fluid coating remains the most practicable and effective preventive measure that can (and therefore should) be taken. The issue of the best material for the job remains unsettled - all that can be said is that experience has shown that both air tool oil and gun oil appear to work pretty well, as does INOX MX3 or equivalent. Vaseline and Cosmoline also appear to be excellent materials for the task. There may well be other materials that are equal or superior to these products. If so, I'd love to hear about them! Probably the ultimate approach is to store the engine in a dessicated environment which is physically isolated from contact with the surrounding atmosphere, as suggested by Jerry. Sealed air-tight containers containing silica gel dessicant would probably work well, as might vacuum sealing along the lines used in food preparation. However, the latter approach would of course greatly complicate the periodic use of the engine, while both approaches would preclude its proper display. For engines which may be displayed or even used from time to time, the "oil in baggie" or silica gel in Ziplok approaches are doubtless the best. Accordingly, I'll be sticking to my "paint in oils" approach, for now at least, although I may well give both INOX and Vaseline a try after reading the above comments from others. I may also have another go using gun blue. If any reader has some better ideas, please share them - all input will be fully acknowledged! __________________________________ Article © Adrian C. Duncan, Coquitlam, British Columbia First published July 2015 |
| |
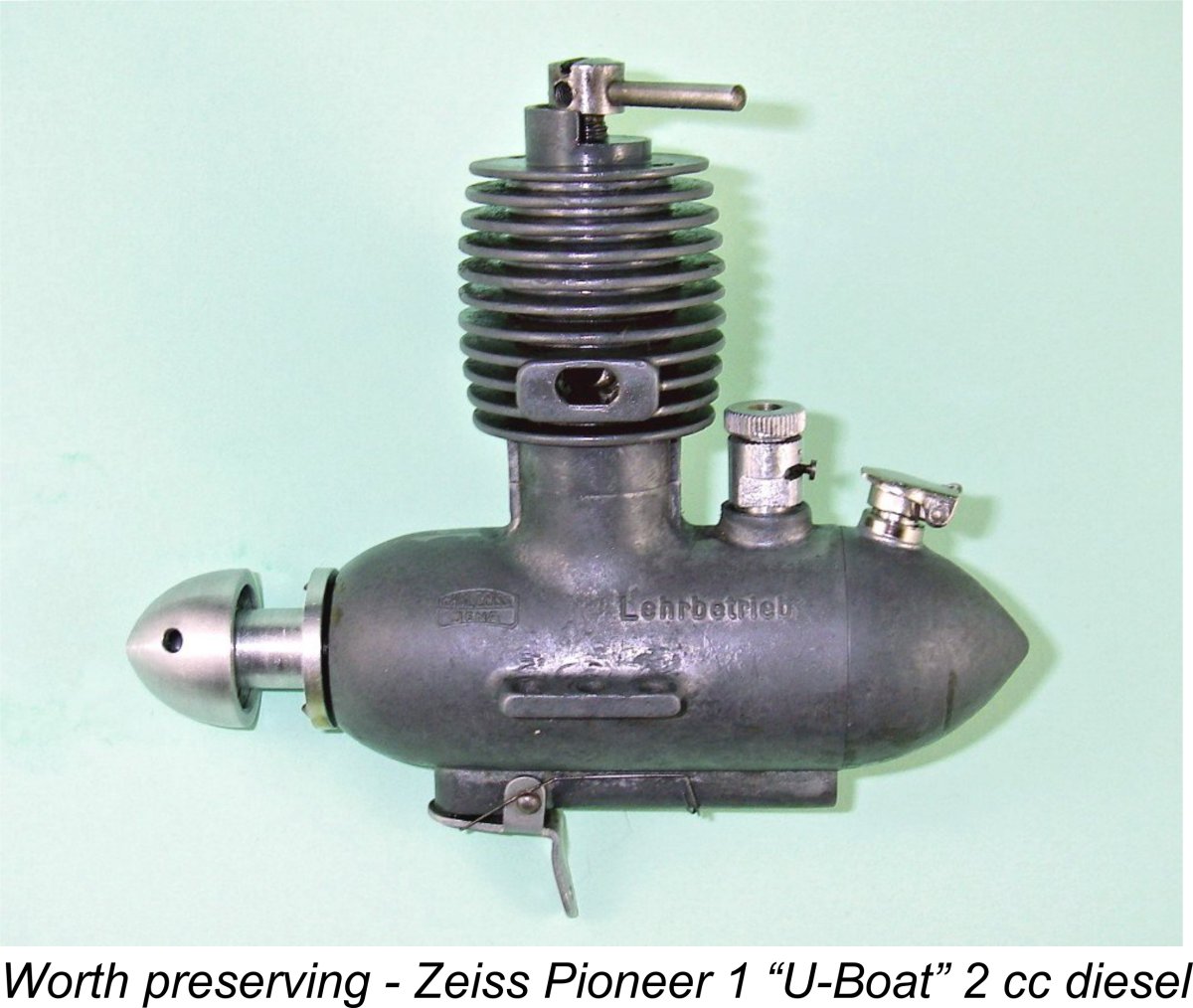 Most enthusiasts who take an interest in classic model aero engines are familiar with at least one or two such motors which are constructed around magnesium alloy castings. During the 1940’s and 1950’s this was quite a popular material for the production of such components as die-cast crankcases, backplates and cylinder heads.
Most enthusiasts who take an interest in classic model aero engines are familiar with at least one or two such motors which are constructed around magnesium alloy castings. During the 1940’s and 1950’s this was quite a popular material for the production of such components as die-cast crankcases, backplates and cylinder heads. A number of present-day formulations are responsive to heat treatment. The strength-to-weight ratio of precipitation-hardened magnesium alloys is comparable with that of the stronger alloys of aluminium or with the alloy steels. Magnesium alloys, however, have a significantly lower density, withstand greater column loadings per unit weight and have a higher specific modulus of elasticity (i.e., enhanced stiffness). They can be used when high structural strength is not the primary requirement but where a light, accurate and
A number of present-day formulations are responsive to heat treatment. The strength-to-weight ratio of precipitation-hardened magnesium alloys is comparable with that of the stronger alloys of aluminium or with the alloy steels. Magnesium alloys, however, have a significantly lower density, withstand greater column loadings per unit weight and have a higher specific modulus of elasticity (i.e., enhanced stiffness). They can be used when high structural strength is not the primary requirement but where a light, accurate and  needs to be done! Since we can’t do anything to the engine’s structure to deal with either form of corrosion, it’s surely obvious that we need to take steps to isolate the magnesium surface from any possibility of direct contact with any form of electrolyte. If that is done, neither form of corrosion can occur.
needs to be done! Since we can’t do anything to the engine’s structure to deal with either form of corrosion, it’s surely obvious that we need to take steps to isolate the magnesium surface from any possibility of direct contact with any form of electrolyte. If that is done, neither form of corrosion can occur.
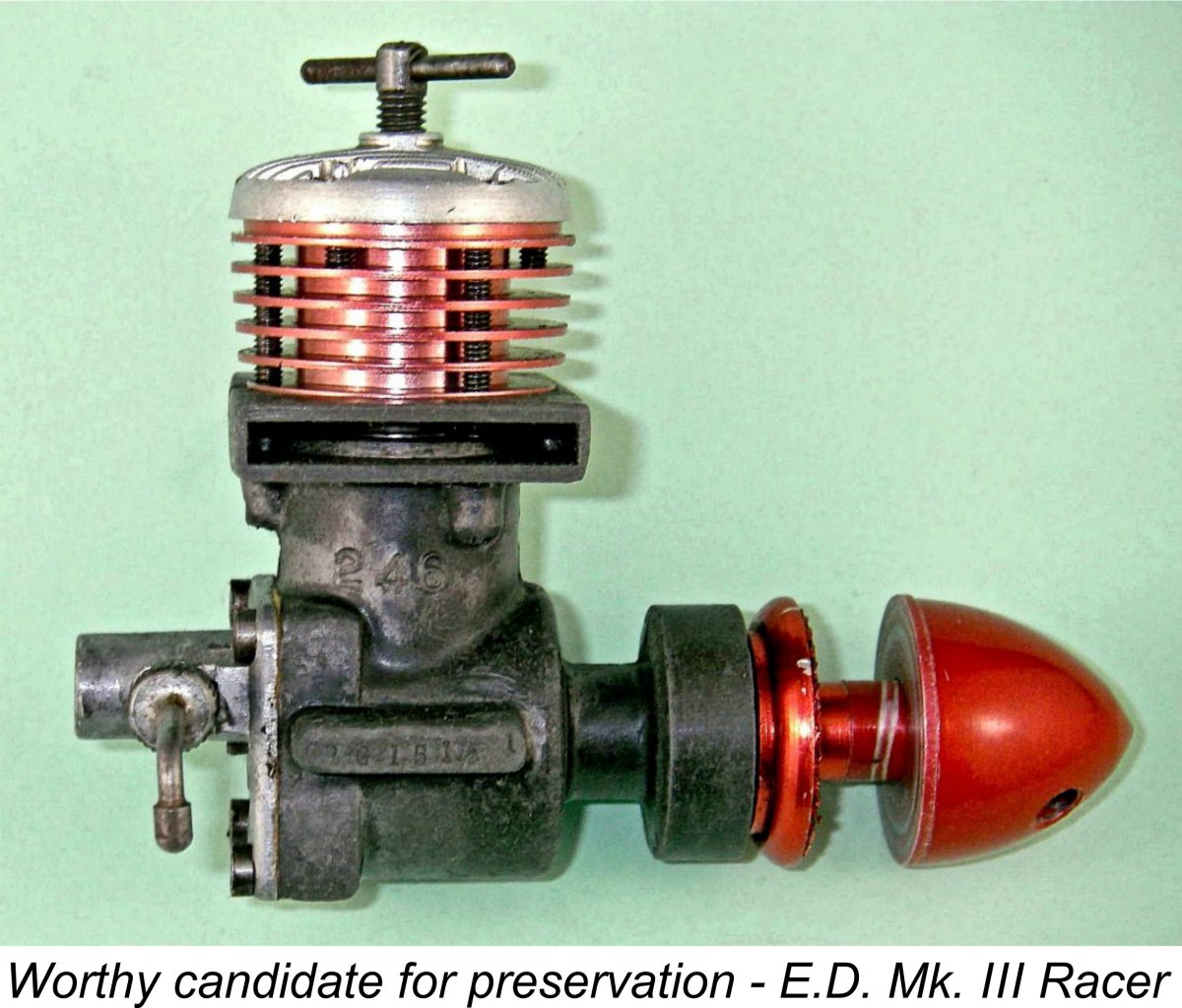 When it comes to cleaning magnesium castings, the key thing is always to be mindful of the high reactivity of magnesium, which requires you to be very careful when choosing a cleaning medium. Beginning with my own experiences, I've found up to now that the best approach is to use an old medium or hard-bristle toothbrush which is lubricated with whatever moisture-displacing oil I'm going to use for storage (in my case air tool oil). This won't cause any marring of the surface, but it will generally see off any residues. Just keep on scrubbing until it's gone! If the engine is corroded internally, you'll probably have to take it apart to deal with the issue in a thorough manner. Don't forget to check those tapped holes for the assembly screws - prime locations for galvanic corrosion!
When it comes to cleaning magnesium castings, the key thing is always to be mindful of the high reactivity of magnesium, which requires you to be very careful when choosing a cleaning medium. Beginning with my own experiences, I've found up to now that the best approach is to use an old medium or hard-bristle toothbrush which is lubricated with whatever moisture-displacing oil I'm going to use for storage (in my case air tool oil). This won't cause any marring of the surface, but it will generally see off any residues. Just keep on scrubbing until it's gone! If the engine is corroded internally, you'll probably have to take it apart to deal with the issue in a thorough manner. Don't forget to check those tapped holes for the assembly screws - prime locations for galvanic corrosion!  A point made by Jerry which might easily be overlooked is that unless you are very sure on the basis of authoritative information that the parts to be cleaned are indeed magnesium alloy, it might be wise to confirm this before embarking upon any cleaning program. This is particularly critical if you plan to use alkaline cleaning agents (see below), since they will generally attack aluminium alloy. To determine the part's density, it can be weighed in air to determine its mass and then in water to determine its volume. From this information, the density can be calculated very easily. The density of a typical magnesium alloy is 1.8 grams per cubic centimeter, while aluminum alloys generally run around 2.7 grams per cubic centimeter.
A point made by Jerry which might easily be overlooked is that unless you are very sure on the basis of authoritative information that the parts to be cleaned are indeed magnesium alloy, it might be wise to confirm this before embarking upon any cleaning program. This is particularly critical if you plan to use alkaline cleaning agents (see below), since they will generally attack aluminium alloy. To determine the part's density, it can be weighed in air to determine its mass and then in water to determine its volume. From this information, the density can be calculated very easily. The density of a typical magnesium alloy is 1.8 grams per cubic centimeter, while aluminum alloys generally run around 2.7 grams per cubic centimeter.

 the same with a mag case engine that has been run, except that I first rinse it thoroughly inside and out with Varsol (an industrial solvent which doesn't affect the metal) to remove any combustion residues before proceeding to the oil injection step. Castor oil residues are not a problem when it comes to preservation, since they actually form a very effective barrier against moisture and corrosion. You'll never find any corrosion under all that gum!
the same with a mag case engine that has been run, except that I first rinse it thoroughly inside and out with Varsol (an industrial solvent which doesn't affect the metal) to remove any combustion residues before proceeding to the oil injection step. Castor oil residues are not a problem when it comes to preservation, since they actually form a very effective barrier against moisture and corrosion. You'll never find any corrosion under all that gum! My late valued friend and colleague David Owen advised that he cleaned any white deposits from the engine and then coated the surface with a lubricant called
My late valued friend and colleague David Owen advised that he cleaned any white deposits from the engine and then coated the surface with a lubricant called  As far as preservation is concerned, Kevin used a slightly different approach to those described earlier. To preserve his cases, he slathers Vaseline (petroleum jelly) all over them, then warms the engine with a hair dryer until the jelly starts to melt. At that point, he brushes it in with a soft toothbrush. This should be repeated at least once a year.
As far as preservation is concerned, Kevin used a slightly different approach to those described earlier. To preserve his cases, he slathers Vaseline (petroleum jelly) all over them, then warms the engine with a hair dryer until the jelly starts to melt. At that point, he brushes it in with a soft toothbrush. This should be repeated at least once a year. I suppose one could sum up by stating that there doesn't seem to be any single magic formula for cleaning and preserving magnesium case model engines. The one common factor appears to be the requirement that whatever preservative treatment is adopted must have the effect of creating a water-resistant barrier between the metallic surfaces and the surrounding atmosphere or any other water source.
I suppose one could sum up by stating that there doesn't seem to be any single magic formula for cleaning and preserving magnesium case model engines. The one common factor appears to be the requirement that whatever preservative treatment is adopted must have the effect of creating a water-resistant barrier between the metallic surfaces and the surrounding atmosphere or any other water source.