
|
|
Fuels for Variable Compression Model Diesels
That said, it's perhaps worth taking a brief side-trip at this point to recall the pioneering efforts of Gustav Eisfeld in Germany. Beginning in 1937, a year prior to the appearance of the ETHA engines in Switzerland, Eisfeld began experimenting with true diesel engines suitable for model aircraft use. Before the end of 1937 he had a 15 cc model diesel operating successfully with an adjustable high-pressure fuel pump, a miniature fuel injector and a compression ratio of 22:1. By 1942 he had refined his design to the point where a 3.7 cc true diesel suitable for model aircraft was being successfully tested. All of these units operated on conventional road diesel fuel (DERV). However, that was as far as it went - at this point the technical impracticability of manufacturing the required ultra-precise miniature injection equipment in quantity forced Eisfeld to return to the more familiar and far simpler carburettor-equipped arrangement in his production models. Although he had amply demonstrated the practicability of constructing miniature true diesels, the technical challenge of manufacturing injector systems at model scales remains just as daunting today as it did back then. Hence all of our model "diesel" engines are actually conventional carburettor-equipped internal combustion units which just happen to use compression ignition instead of some form of point ignition source (spark or glow-plug). All two-stroke engines (and model four-stroke engines) require two key elements in their fuel - the base fuel which burns in the cylinder and provides the power, and a lubrication component. Model diesels are no different, but they require some additional components solely to aid the ignition process. Before I go into the specifics of model diesel fuel, I wish to add a strong word of caution. I do NOT recommend that modellers try to mix their own diesel fuels! I do it myself, but that is a personal decision which affects no-one but myself. That decision is taken in light of a comprehensive understanding of the materials involved and their associated hazards. Anyone who is tempted to try mixing their own fuel should make themselves similarly well-informed prior to making any decision to proceed. Any such decision must be taken entirely on their own recognizance in the full light of a clear understanding and conscious acceptance of the hazards.
Sadly, this situation has not lasted. The ever-increasing restrictions on the availability of a number of primary ingredients of model engine fuels (ether, castor oil and most recently nitromethane), has resulted in most of the commercial suppliers abandoning the field. The overwhelming worldwide adoption of the completely soul-less brushless electric technology has greatly reinforced that trend by substantially reducing the overall demand for model I/C engine fuels, making that line of business progressively less economically viable. As the fuel suppliers drop out, many of us have been left on our own ……….. My purpose here is strictly to provide an understanding of the roles of the various constituents of model diesel fuel so that modellers retaining an interest in using diesels can appreciate what is going on in their engines and make informed decisions about the suitability of a given fuel mix for use in those engines. Anyone wishing to consider mixing their own fuel should read and understand the following material in its entirety before making any such decision. Before we dive into the subject of current formulations for model diesels, it’s actually quite instructive to take a quick look back and see Although ether was already widely recognized as an essential component of any practicable model diesel fuel, the range of suggested base fuel components went from naphthalene through turpentine to ordinary petrol (gasoline). Kerosene (or paraffin if you prefer) was conspicuous by its absence from most mixtures - its role was seen at the time as being confined to reducing the well-recognized tendency of ether to detonate (or knock, if yer a Brit!). Ordinary petrol (gasoline) was the most commonly-recommended base fuel. This was likely a carry-over from spark ignition days. With a couple of exceptions, castor oil was also a notable absentee from this table. This too was likely a carry-over from spark ignition operation, in which SAE 70 mineral oil had long been the accepted standard, as it remains today if you can get it. Finally, none of the mixtures recommended by the listed manufacturers included any ignition improver such as amyl nitrate. This is perhaps a little odd, since published research undertaken by Lawrence Sparey and other members of the staff of "Aeromodeller" magazine had already identified ethyl nitrate as an effective ignition improver. However, the use of organic nitrates for this purpose had apparently not yet filtered through to the manufacturers. By the late 1940's this had changed, largely as a result of the rapid development of rotary valve engine designs which were capable of far higher operating speeds than their sideport predecessors. Kerosene had become the standard base fuel, while castor oil had become the lubricant of choice. Both of these materials retain their prominence today. The most important step forward, however, was the general adoption of the use of a small percentage of an ignition improver such as amyl nitrate. This greatly improved running characteristics, particularly at high speeds. More of its function below ................ Thus by 1950 the basic diesel fuel formulation still in use today had become well established. Time now to look into the characteristics and function of the various materials involved. Briefly, a typical model diesel fuel has four major ingredients, which will be discussed in turn.
Let's look at each of these in more detail.
Reader Leonard Poon of New Zealand contacted me to advise that he had been using locally-produced bio-diesel fuel as a 100% replacement for the kerosene content in his home-brewed model diesel fuels. I'm not surprised - after all, kerosene has very similar combustion characteristics as regular road diesel fuel, and bio-diesel is intended to be used as a substitute fuel in the same full-sized diesel engines with no modification. If I could find a local source, I'd give it a try myself. Leonard told me that the bio-diesel fuel that he used as a kerosene substitute was locally produced from a base of used cooking oil. Apart from the tiny environmental benefit, a great advantage of using this material is the fact that there is very little exhaust odour on the engine afterwards - none of that "burned diesel" smell that less sympathetic folk sometimes object to! I reckon that this is well worth a try if you can find a local source. How much kerosene can or should be used? The more kerosene in the mix, the greater the power potential of the fuel, but more kerosene inescapably means less ether. At some point, you get into a situation where the extra power benefits become subsidiary to the resulting starting difficulties and increased compression requirements. Between 40% and 45% appears to be the optimum range based on my many years of experience.
There's a lot of controversy about the best kind of oil to use in model diesels and indeed in model engines in general. I've discussed this topic in great detail in a separate article about Model Engine Lubricants to be found elsewhere on this web-site. Accordingly, I'll confine myself in this article to a brief summary.
Thirdly, the operation of an engine invariably results in the continuous generation of microscopic particulates, both metallic and combustion-related. The oil is the one component of the fuel which in theory does not burn, being ejected in a steady stream out of the exhaust as long as the engine is running. In doing so, it carries away the particulates from within the engine, thus continuously purging them from the system and preventing any build-up. An oil that burns more readily is less effective in this role than one which does not burn readily. A non-operational conservation issue arises from the fact that all the other fuel components are burned as the engine runs, in some cases leaving corrosive residues. The oil is the primary residual component when the engine stops. Therefore, it also has to guard against post-operative corrosion. When considering the role of oil in fulfilling the first two requirements set out above, we need to bear in mind that the very high compression ratios at which model diesels run (compared to glow-plug or spark-ignition engines) combine with their more “explosive” ignition characteristics to create significantly higher gas pressures, particularly around top dead centre. This in turn places far greater loads on the bearings and other wearing surfaces, imposing substantially greater demands on the oil both as a high pressure lubricant and as a sealant. A model diesel is a real test of any lubricant!
The undeniable tendency of castor oil to polymerize into gummy residues during long-term engine storage makes the use of a good after-run oil very advisable. However, castor oil gum is an excellent corrosion inhibitor - you'll never find rust under all that gumminess! I can already hear the vehement arguments from oil manufacturers, fuel blenders and even some users in favour of synthetic oils. However, the proof lies both in the scientific evidence and in the every-day real-world experiences of long-term diesel users like me. My colleagues and I have in fact experimented extensively with state-of-the-art synthetic-based mixes, experiencing a range of problems ranging from hard starting through accelerated engine wear to engine overheating and even seizure. A particular issue with synthetics is a general failure to provide a sufficient high-temperature piston/cylinder seal. My advice - stick firmly to castor oil or heavy mineral oil, and don't accept any substitutes! If you want to know more about my reasons for holding this view, please refer to my lubrication article mentioned earlier. To be suitable for model engine lubrication purposes, castor oil has to be of That said, reader Leonard Poon of New Zealand challenged my views, stating that he had been using medicinal "Castor Oil B.P." with complete satisfaction in his P.A.W. and Sharma diesels. Leonard also noted that P.A.W. themselves recommend the use of "Medicinal Castor Oil" in the leaflets supplied with their current engines. Perhaps it all depends upon the particular product that you use ........I may give it another try sometime, using an "expendable" engine. Because of the Ricin scare, castor oil specifically intended for model engine purposes has become far harder to get in recent years (once again, legitimate users pay for the misdemeanours of the criminal set). Fortunately, suitable 100% pure cold-pressed castor oil remains readily available (for now, at least) through health food and beauty stores, where it is sold as a skin conditioner. It costs a little more than the oil formerly sold in hobby shops, but the results are well worth the cost. As far as the desirable oil content goes, long experience shows that for most purposes around 25% seems to represent the best compromise between lubrication and sealing requirements. More than this appears unnecessary - indeed, a further increase in the oil content will reduce the power-generating potential of a given amount of fuel by displacing the power-generating components from the mix. However, much less than this seems to affect engine life over the long term. Lower oil contents also give rise to increasing sealing problems as engines become more worn, particularly in diesels used for such purposes as vintage combat where dirt ingestion is a fact of life. Having said this, it’s only fair to point out that when maximum performance and fuel economy are primary criteria (as in team racing), the oil content may be reduced considerably below the 25% figure to allow for the inclusion of additional power-generating components. A well-fitted and carefully run-in team race diesel will give several hours of top performance on such a low-oil mix before wear begins to create sealing problems. A higher wear rate is the price to be paid for increased performance and better fuel economy. You can't have it all! Despite the superiority of castor oil, it's worth pointing out once again that lower-performance sports diesels, particularly sideport units, can be operated perfectly satisfactorily on fuels based on heavy mineral oil. Such fuels have the great advantage that their residues do not "gum up" during storage. I use such fuels myself routinely in my classic sideport diesels. Even with engines which are run using a castor-based fuel, I often run a tank of mineral-based fuel through the engine at a low compression setting if I'm planning to store it for an extended period. SAE 50 or SAE 60 mineral oils (AeroShell 100 or AeroShell 120) work very well, while SAE 20/50 multigrade also appears to work well if the others are not available. One potential difficulty with castor oil is that it is insoluble in kerosene. This would preclude its use in model diesel engines were it not for the fortunate fact that both castor oil and kerosene are mutually soluble in ether! Hence in part the reason for the next ingredient to be discussed.
That said, it must be admitted that model diesels have been run successfully on etherless fuels. Indeed, in places like wartime Norway where pioneers like Jan David-Andersen were conducting initial experiments with model diesels, etherless fuels were used out of necessity since ether was simply unavailable. There have been a number of successful trials in more recent times as well. However, such fuels require a significantly higher compression ratio, which places undue stresses upon engine components while also increasing pumping power losses and creating starting challenges. Far better to keep adding the ether if it's available ........... The second great benefit of using ether is the fact that it has an unusually wide range of explosive limits – that is, the range of percentages of ether vapour in air which will ignite and burn. For ether, the range is between 1.9% and 36% of vapour in air – far wider than most other fuel components. To give you some idea, the comparable range for kerosene is 0.7% to 5% - nowhere close to ether. This means that an ether-based fuel will ignite over a very wide range of fuel mixtures as set at the needle valve. The resulting flexibility is a very good thing in terms of model diesel operation. The third extremely positive attribute of ether requires some preliminary discussion. It’s a widely unappreciated fact that air-cooled two-stroke internal combustion engines are in reality not air-cooled at all – they’re fuel-cooled! As the fuel is atomized in the carburettor, it moves directly from the liquid to the vapor state. In doing so, it extracts heat from the incoming air, thus cooling it. The extent to which it does this is determined by a property of the fuel called the Latent Heat of Vaporization. For a given fuel constituent, this is the amount of energy absorbed by a give quantity of that constituent when changing from the liquid to the vapor state.
It was for this reason that years ago when I was road-racing air-cooled Bultaco two-stroke motorcycles, I always jetted for optimum engine temperatures rather than all-out power. I may have sacrificed the odd horsepower, but the reliability of these "Spanish hand grenades" in my hands was legendary! To finish first, you first have to finish …………… In the case of our model diesel engines, ether has a relatively high latent heat of vaporisation. In particular, the standard figure for ether of 377 Kj/kg is 50% higher than the corresponding figure for kerosene (251 Kj/kg). This means that the ether content in the fuel considerably enhances the fuel’s ability to cool the incoming air flow and thus internally cool the engine itself. The ether thus plays a key role in controlling engine operating temperatures. Fourth and finally, ether is an excellent solvent. Since castor oil is insoluble in kerosene, a solvent is required to allow the fuel ingredients to mix. Ether fulfills that function to perfection. Pretty convenient all round, eh?!? However, at this point the advantages end. It is important to understand that, for all of its desirable qualities set out above, ether is a bad diesel fuel in its own right. It has a lower calorific value than the kerosene which it displaces, releasing only 43,000 kilojoules of energy per kilogramme (Kj/kg) compared with 46,200 Kj/kg for kerosene. Consequently, it contributes less to the power-generating potential of the fuel. More problematically, it tends to detonate (explode) unpredictably rather than auto-ignite smoothly and cleanly. Since detonation places enormous shock loadings on engine components, it is a condition to be avoided at all costs. For these reasons, the amount of ether used in a given fuel should be no more than that required to allow the ether to fulfil its compression-reduction and solvent roles. In summary, the ether's sole functions in a model diesel are; to lower the temperature at which ignition of the fuel mixture will commence (and hence lower the required amount of compression required for starting and running); to aid in the internal cooling of the engine; and to keep all the ingredients of the fuel in solution. We need to use just enough ether to meet all of these requirements, and no more!
Ether is also extremely volatile, hence evaporating readily. Accordingly, model diesel fuel should always be kept in a well-sealed metal container except when being transferred to a field container or to the model. Finally, ether is highly reactive, hence being an excellent solvent. You’ll soon learn this if you try building a foam-core model for your diesel powerplant and fail to fuel-proof it very thoroughly indeed – it’s the ether which will quickly and efficiently dissolve your lovely foam core!! An often-overlooked characteristic of ether, but one which can kill the unwary, is the fact that it can form highly unstable peroxides if exposed to light for any length of time. Both straight ether and mixed diesel fuel should therefore be stored solely in well-sealed metal containers in a cool and dark location. The tendency to form peroxides is significantly reduced once the ether is in solution with the other ingredients of diesel fuel, but the danger is still there regardless. In terms of the proportion of ether which should be incorporated into a model diesel fuel, it’s worth noting that the pioneers mentioned at the start of this article were quite correct in believing that the kerosene content in a model diesel fuel greatly reduces the tendency of the ether to detonate. Consequently, we do have some leeway to play with. Long experience shows that between 25% and 30% ether appears to be the optimum range for most applications. Mixtures at the higher end of this range are advantageous in that they contain some allowance for the inevitable evaporation of some of the ether as the fuel is used. The one exception to this general rule is in the case of very small diesel engines – those of 0.5 cc (0.030 cuin.) displacement or less. Such engines seem to require a higher ether content than their larger cousins – a figure of 40% or more is not uncommonly required for best results with these little gems. The kerosene content is reduced to make room for the extra either – the oil content should remain unchanged. Anyone considering mixing their own fuel (a procedure which I do NOT recommend to others) will quickly discover that as in the case of castor oil, the activities of the criminal sub-culture have once again infringed upon the freedom of legitimate users to obtain ether. In recent years, the ready availability of this material has become increasingly compromised, largely because of its use as a solvent in the making of illicit drugs such as crystal meth. The most accessible source at present is the automotive “starting fluid” available from many automotive or heavy equipment supply stores. The better grades of this material have a high ether content in the 60% plus range, with the bulk of the balance being heptane in most cases. Heptane is actually a member of the ether family in a chemical sense, although it has a somewhat higher auto-ignition temperature (204 deg. C as opposed to 160 deg. C for ether). Its combustion characteristics are however far closer to those of ether than those of kerosene in that it tends to burn more “explosively” than kerosene. Regardless, the presence of a proportion of heptane in the mix does not appear to greatly affect the performance of an either-based diesel fuel as long as the ether predominates.
When using John Deere starting fluid, I do so on the basis that it is essentially pure ether, ignoring the 20% content of additional ingredients. I make no allowance for those ingredients, simply using the starting fluid in the same proportion that I would for pure ether. However, when using starting fluids having a lower ether content, I make some allowance for the additional ingredients. Using Gunk Liquid Fire, for instance, I measure the volume extracted from the can and multiply that volume by 0.7, thus making allowance for the presence of 30% non-ether content. The volume obtained after the calculation is taken as the volume of ether which that can actually represents. I then base the balance of my mix on the representative volume of ether derived from my calculation. The actual volume of starting fluid is of course 30% greater, but I allow for that by reducing the kerosene content to give the correct proportion of ether to the other combustible ingredients. This is fine, because the heptane which thus replaces the displaced kerosene has broadly similar combustion properties, including an almost identical calorific value. I then add the oil and ignition improver (see below) and I’m set! Fuel made in this way performs in a completely satisfactory manner for most purposes. As an example, here are the calculations for a batch of fuel made to my usual specifications for vintage diesel combat using Gunk Liquid Fire:
In my personal experience, batches of fuel mixed on this basis using Gunk Liquid Fire perform in a completely satisfactory manner. Different starting fluid formulations will require the use of a different factor for calculating the pure ether equivalent (step 2 above), but the principles remain the same. Starting fluid is sold in an aerosol spray can, and my own method of extraction for home mixing is very simple. I begin by holding the can upside down and pressing the spray button to open the valve. The pressure bleeds off while the liquid contents remain in place inside the can. This process needs to be repeated several times with a pause between attempts, since part of the content consists of dissolved gasses which take time to come out of the ether. Once the pressure has been completely relieved and repeated presses produce no more hissing, I then take a sharp-ended camping can-opener (the kind with a triangular blade at one end) and cut a small hole in the base of the de-pressurized aerosol can. This releases any remaining pressure in the system. I then cut a second larger opening on the Please note that in providing this information, I am NOT recommending that others attempt to mix their own fuel using such materials or methods. In particular, I DO NOT claim that this is necessarily a safe technique, NOR do I recommend that anyone else use it in preference to any other method of extraction (or none at all). On the contrary, I wish to state very clearly for the record that the application of such techniques is potentially VERY DANGEROUS and could result in injury or even death. The approach described has worked well for me up to now, and I’m still here to prove it......but perhaps I've just been lucky so far! Others may or may not wish to try this technique themselves – if so, it’s ENTIRELY at their own discretion and at their own risk based on a clear understanding that injury or death could result from employing such procedures. YOU HAVE BEEN CAUTIONED!! End of liability disclaimer…….
This is the one component that approaches the realm of "black magic" in terms of model diesel fuel technology. It is a relatively minor component which has the effect of reducing the lag time for ignition to commence once the fuel's auto-ignition temperature (the temperature required for spontaneous ignition to occur in the absence of any ignition source) is reached. Fuels having reduced ignition lag times are said to have enhanced Cetane ratings. The issue of ignition lag is widely unappreciated by present-day model diesel users. As mentioned previously, ether has an auto-ignition temperature of only 160 degrees Celcius. However, when that temperature is reached in the cylinder, the ether does not ignite immediately - there is a short but nontheless measurable lag time between the achievement of the required temperature and the actual commencement of ignition, during which time the piston is of course continuing to rise against compression. The result of this is that to achieve a given ignition timing, the compression has to be set at a slightly higher level than that theoretically required for the auto-ignition temperature of the fuel to be reached. As speeds increase, the amount of extra compression required naturally increases. The presence of an ignition improver reduces the ignition lag time appreciably, thus contributing to more precise ignition timing along with enhanced combustion characteristics. As a result, high-speed performance is greatly improved, an effect which is readily meaurable by the engine's user. Apart from this, the most readily observable evidence for the reduction in the ignition lag time arising from the use of ignition improver is the accompanying reduction in the compression requirements for starting and running, which in turn reduces both pumping energy losses and stresses on the working components. However, as is so often the case, there is a price to be paid for these positive effects. The addition of an ignition improver tends to make the engine run hotter, hence having a greater tendency to sag as it reaches full operating temperature. For this reason, only a small percentage of ignition improver is required - too much of such a constituent causes overheating, sagging and erratic running.
In general, the materials used for this purpose are organic nitrates or nitrites. The most commonly-used ignition improvers in the past have been amyl nitrate (mostly in North America since the 1970’s), amyl nitrite and iso-propyl nitrate (commonly used in Europe in more recent decades). All of these substances are highly volatile, semi-explosive materials which require very respectful handling in their pure state. However, they become relatively stable once in solution with other fuel ingredients.
The alternative material amyl nitrite is extremely dangerous from a health standpoint - it's used as a heart stimulant in medicine but can kill you if improperly used - a number of "recreational" users have found this out the hard way! The material is a coronary vasodilator, hence its medical application. However, if improperly used it can cause over-elevated heart rate, headache and dizziness together with a sharp decrease in blood pressure with a resulting loss of consciousness. It may also cause methemoglobinemia, which affects the ability of the bloodstream to deliver oxygen to the body's tissues. It is characterized by dizziness, drowsiness, headache, shortness of breath, cyanosis (bluish discoloration of the skin due to deficient oxygenation of the blood), rapid heart rate, unconsciousness and ultimately death. Not good stuff with which to mess around! A seemingly less potentially harmful alternative which remains currently (2024) available in North America is Amsoil Cetane Boost additive for full-sized diesel fuels. This is 100% octyl nitrate, which works just about as well as the amyl variety. It is available commercially from local Amsoil dealers. As mentioned earlier, the benefits of these improvers are most marked over the first few percent in the mix. After that, additional amounts tend to cause erratic running, excessive overheating and sagging following warm-up. About 2½% seems to be the limit for amyl nitrate and octyl nitrate, and around 2% for iso-propyl nitrate. Above these percentages, the downsides noted above begin to put in an appearance. One of the downsides of being a diesel user is "the smell"! We die-hard diesel users love it, but others don’t!! Actually, much of this is caused by the amyl nitrate or nitrite in the fuel. Iso-propyl nitrate does not create anywhere near the same "aromatic" issue, so it's a pity that it does not seem to be generally available to North American blenders. If you ever go to Britain to fly your diesels, try some of their iso-propyl-based fuels - you'll be pleasantly surprised! Well, there it is! That's what I've learned about model diesel fuels over the last 65 years or so (yes, I’m that old!!). My basic mix for nostalgia diesel combat is: Kerosene - 42.5%
In extremely hot weather, I find that the addition of a few extra percent of castor oil to the above mix can work wonders for consistency and dependability. It also helps in starting a worn engine by improving the piston seal. This is the only adjustment that I ever need to make - the above mix generally works well under the full range of conditions that we usually encounter in our geographic area. For the really small diesels, such as the Allbon Bambi 0.15 cc or the K Hawk 0.196 cc seen at the left, I find that a little more ether really helps with starting. Some extra oil also seems to be warranted, both to protect the tiny parts from wear and to promote a really good compression seal. My standard mix for the Bambi and similar miniatures is: Kerosene - 27.5% Finally, for vintage sideport diesels running at speeds below 8,000 rpm, I generally use a straight un-nitrated fuel with lots of oil: Kerosene - 35% In such engines, a heavy mineral oil (SAE 60 or SAE 70) may be used in place of castor oil. I hope that the above information will help you select the best fuel mixture for your own needs. Good flying! _______________________ Article © Adrian C. Duncan, Coquitlam, British Columbia, Canada First published June 2015 Updated October 2018 |
| |
 The so-called diesel engines used in modelling are actually not true diesels at all, since they don't have a fuel injection system but function exactly like a full-size two-stroke engine, the only difference being that they rely solely on compression-generated heat for ignition. They are thus more correctly characterized as compression-ignition two stroke engines (or four stroke according to
The so-called diesel engines used in modelling are actually not true diesels at all, since they don't have a fuel injection system but function exactly like a full-size two-stroke engine, the only difference being that they rely solely on compression-generated heat for ignition. They are thus more correctly characterized as compression-ignition two stroke engines (or four stroke according to  As of my initial time of writing (mid 2015), good model diesel fuels remained commercially available in a number of countries, in some cases prepared to customer-specified formulas. The materials involved are both hazardous to handle and potentially injurious to your health. Moreover, some of them are restricted substances, hence being a hassle to get hold of. Finally, the cost of such ready-mixed fuels was often less than that of home-brewing. Fuel mixing was therefore best left to the professionals if that option was open to you ………..
As of my initial time of writing (mid 2015), good model diesel fuels remained commercially available in a number of countries, in some cases prepared to customer-specified formulas. The materials involved are both hazardous to handle and potentially injurious to your health. Moreover, some of them are restricted substances, hence being a hassle to get hold of. Finally, the cost of such ready-mixed fuels was often less than that of home-brewing. Fuel mixing was therefore best left to the professionals if that option was open to you ……….. 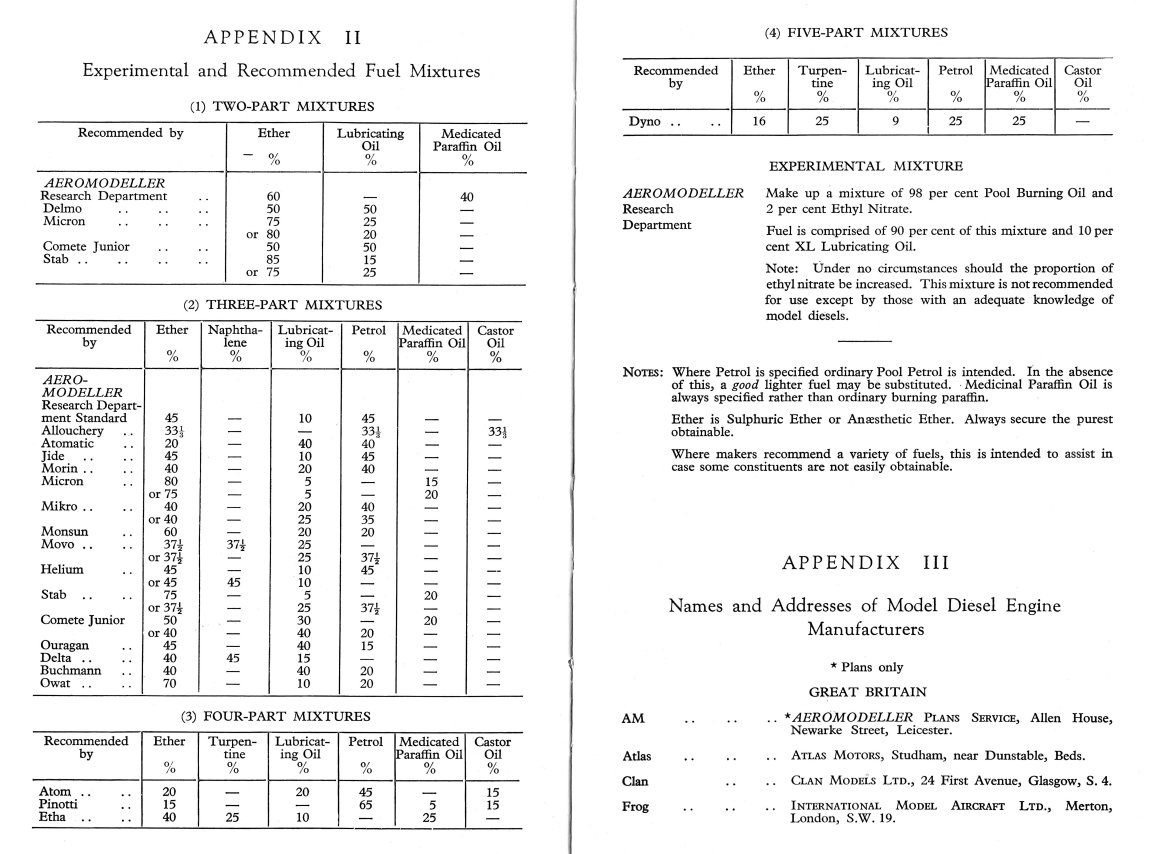 how we got here! Appendix II of D. J. Laidlaw-Dickson's ground-breaking late 1946 book "Model Diesels" (reproduced at the left) provides an excellent summary of the range of model diesel fuel mixtures then being recommended by the manufacturers of such engines.
how we got here! Appendix II of D. J. Laidlaw-Dickson's ground-breaking late 1946 book "Model Diesels" (reproduced at the left) provides an excellent summary of the range of model diesel fuel mixtures then being recommended by the manufacturers of such engines.  Kerosene is actually a refined form of the diesel fuel used in full-sized road vehicles, hence having very similar ignition and combustion properties. In fact, road diesel fuel can be used quite successfully as the base fuel in model diesels. However, kerosene has less of an "oily" aroma and burns more cleanly than road diesel fuel, thus being more suitable for model use. It has a relatively high calorific value (the amount of energy released for a given amount burned) and a relatively low auto-ignition temperature (the temperature at which it will spontaneously self-ignite without any initiating flame source). Both of these features make it a very good base fuel for model diesels. However, its auto-ignition temperature (around 220 deg. C) is still too high for our miniature two-strokes to deal with, hence the need for other constituents as discussed below.
Kerosene is actually a refined form of the diesel fuel used in full-sized road vehicles, hence having very similar ignition and combustion properties. In fact, road diesel fuel can be used quite successfully as the base fuel in model diesels. However, kerosene has less of an "oily" aroma and burns more cleanly than road diesel fuel, thus being more suitable for model use. It has a relatively high calorific value (the amount of energy released for a given amount burned) and a relatively low auto-ignition temperature (the temperature at which it will spontaneously self-ignite without any initiating flame source). Both of these features make it a very good base fuel for model diesels. However, its auto-ignition temperature (around 220 deg. C) is still too high for our miniature two-strokes to deal with, hence the need for other constituents as discussed below. It's an often-overlooked fact that the oil in a model engine has three operational functions, not just one.
It's an often-overlooked fact that the oil in a model engine has three operational functions, not just one. Taking all of the above requirements into account, there is no doubt whatsoever in my mind that castor oil remains by far the most effective lubricant for any model diesel. A high-quality heavy mineral oil can certainly be used, but castor oil remains superior. As a lubricant, it has unparalleled film strength both hot and cold. It also maintains adequate viscosity for sealing purposes at all times, even at high temperatures when diluted with other fuel ingredients. It does not burn readily, hence performing its purging function very effectively.
Taking all of the above requirements into account, there is no doubt whatsoever in my mind that castor oil remains by far the most effective lubricant for any model diesel. A high-quality heavy mineral oil can certainly be used, but castor oil remains superior. As a lubricant, it has unparalleled film strength both hot and cold. It also maintains adequate viscosity for sealing purposes at all times, even at high temperatures when diluted with other fuel ingredients. It does not burn readily, hence performing its purging function very effectively. 
 Ether (or more properly diethyl ether, to give it its full name) has a number of properties which combine to make it an essential component of model diesel fuel. Firstly and most importantly, it has an extremely low auto-ignition temperature - the temperature at which it will spontaneously ignite in the absence of any ignition source. The figure of around 160 deg. C for ether is significantly lower than the corresponding temperature of 220 deg. C for kerosene. Hence a fuel containing ether will run at significantly lower compression ratios than one consisting of straight kerosene or road diesel oil. The benefits in terms of ease of starting and engine stress management should be obvious.
Ether (or more properly diethyl ether, to give it its full name) has a number of properties which combine to make it an essential component of model diesel fuel. Firstly and most importantly, it has an extremely low auto-ignition temperature - the temperature at which it will spontaneously ignite in the absence of any ignition source. The figure of around 160 deg. C for ether is significantly lower than the corresponding temperature of 220 deg. C for kerosene. Hence a fuel containing ether will run at significantly lower compression ratios than one consisting of straight kerosene or road diesel oil. The benefits in terms of ease of starting and engine stress management should be obvious. As a result of the atomization process in the carburettor, the fuel mixture entering the crankcase from the spraybar is very cool indeed! Since this mixture swirls around in the crankcase and then moves up the bypass into the cylinder, it has the effect of cooling the interior of the engine. This effect is at least as important as the air-cooling provided by the finned cylinder jacket – probably more so in fact since the surface area exposed to this internal cooling effect is if anything greater and the cooling medium is far colder than ambient air. More importantly still, the effect is not confined to the upper cylinder. The piston in particular benefits greatly, since this is the sole cooling process to which it is directly exposed. A two-stroke engine would quickly overheat and seize were it not for this internal cooling effect.
As a result of the atomization process in the carburettor, the fuel mixture entering the crankcase from the spraybar is very cool indeed! Since this mixture swirls around in the crankcase and then moves up the bypass into the cylinder, it has the effect of cooling the interior of the engine. This effect is at least as important as the air-cooling provided by the finned cylinder jacket – probably more so in fact since the surface area exposed to this internal cooling effect is if anything greater and the cooling medium is far colder than ambient air. More importantly still, the effect is not confined to the upper cylinder. The piston in particular benefits greatly, since this is the sole cooling process to which it is directly exposed. A two-stroke engine would quickly overheat and seize were it not for this internal cooling effect.  Ether is extremely flammable, having a flash point of -40 deg. C. The flash point of a liquid is the lowest temperature at which it can vaporize to form an ignitable mixture in air. The low figure for ether confirms that it can do this at any temperature at which it is likely to be used as a component of model engine fuel. Accordingly, neither pure ether nor mixed diesel fuel should ever be handled anywhere near an external ignition source of any kind. As a matter of fact, it is highly unsafe to store ether or fuel in a refrigerator, since fridges are set at temperatures well above ether’s flash point of -40 deg. C and moreover have a potential ignition source in the form of the switch which activates the interior light when you open the fridge door.
Ether is extremely flammable, having a flash point of -40 deg. C. The flash point of a liquid is the lowest temperature at which it can vaporize to form an ignitable mixture in air. The low figure for ether confirms that it can do this at any temperature at which it is likely to be used as a component of model engine fuel. Accordingly, neither pure ether nor mixed diesel fuel should ever be handled anywhere near an external ignition source of any kind. As a matter of fact, it is highly unsafe to store ether or fuel in a refrigerator, since fridges are set at temperatures well above ether’s flash point of -40 deg. C and moreover have a potential ignition source in the form of the switch which activates the interior light when you open the fridge door. It’s important to realize that not all starting fluids are created equal! Some in fact (like the evocatively-named Australian product “
It’s important to realize that not all starting fluids are created equal! Some in fact (like the evocatively-named Australian product “ opposite side through which the contents can be drained into whatever container is to be used to mix the ingredients of the fuel. Needless to say, I do this outdoors well away from any ignition source! I also wear heavy gloves and face protection, just in case ...............
opposite side through which the contents can be drained into whatever container is to be used to mix the ingredients of the fuel. Needless to say, I do this outdoors well away from any ignition source! I also wear heavy gloves and face protection, just in case ...............  At the higher speeds, the benefits of using an ignition improver clearly outweigh any detriments, but this is not necessarily the case at lower speeds such as those at which sideport diesels of the pioneering era typically operate. At speeds of up to (say) around 8,000 rpm, the issue of ignition lag is far less of a problem than it is at higher speeds because everything is happening at a reduced rate anyway. The avoidance of sag following warm-up becomes paramount in such a case. Consequently, it will often be found that a straight fuel without ignition improver will yield superior results with a low-speed vintage sideport diesel than a more potent blend with some improver.
At the higher speeds, the benefits of using an ignition improver clearly outweigh any detriments, but this is not necessarily the case at lower speeds such as those at which sideport diesels of the pioneering era typically operate. At speeds of up to (say) around 8,000 rpm, the issue of ignition lag is far less of a problem than it is at higher speeds because everything is happening at a reduced rate anyway. The avoidance of sag following warm-up becomes paramount in such a case. Consequently, it will often be found that a straight fuel without ignition improver will yield superior results with a low-speed vintage sideport diesel than a more potent blend with some improver. Here some serious
Here some serious  On the above mix, direct measurements show that a well broken-in and warmed-up diesel will typically run at compression ratios of around 17 or 18 to 1 - quite manageable! If you're needing to use much more than this, chances are there's something wrong with your fuel!!
On the above mix, direct measurements show that a well broken-in and warmed-up diesel will typically run at compression ratios of around 17 or 18 to 1 - quite manageable! If you're needing to use much more than this, chances are there's something wrong with your fuel!!